Exploring Nivolumab's R&D successes and its clinical results at the 2024 ASCO_GU
On 25 Jan 2024, the latest clinical data from the subcutaneous nivolumab (NIVO SC) vs intravenous nivolumab (NIVO IV) in patients with previously treated advanced or metastatic clear cell renal cell carcinoma (ccRCC) was reported in 2024 ASCO_GU.
Nivolumab's R&D Progress
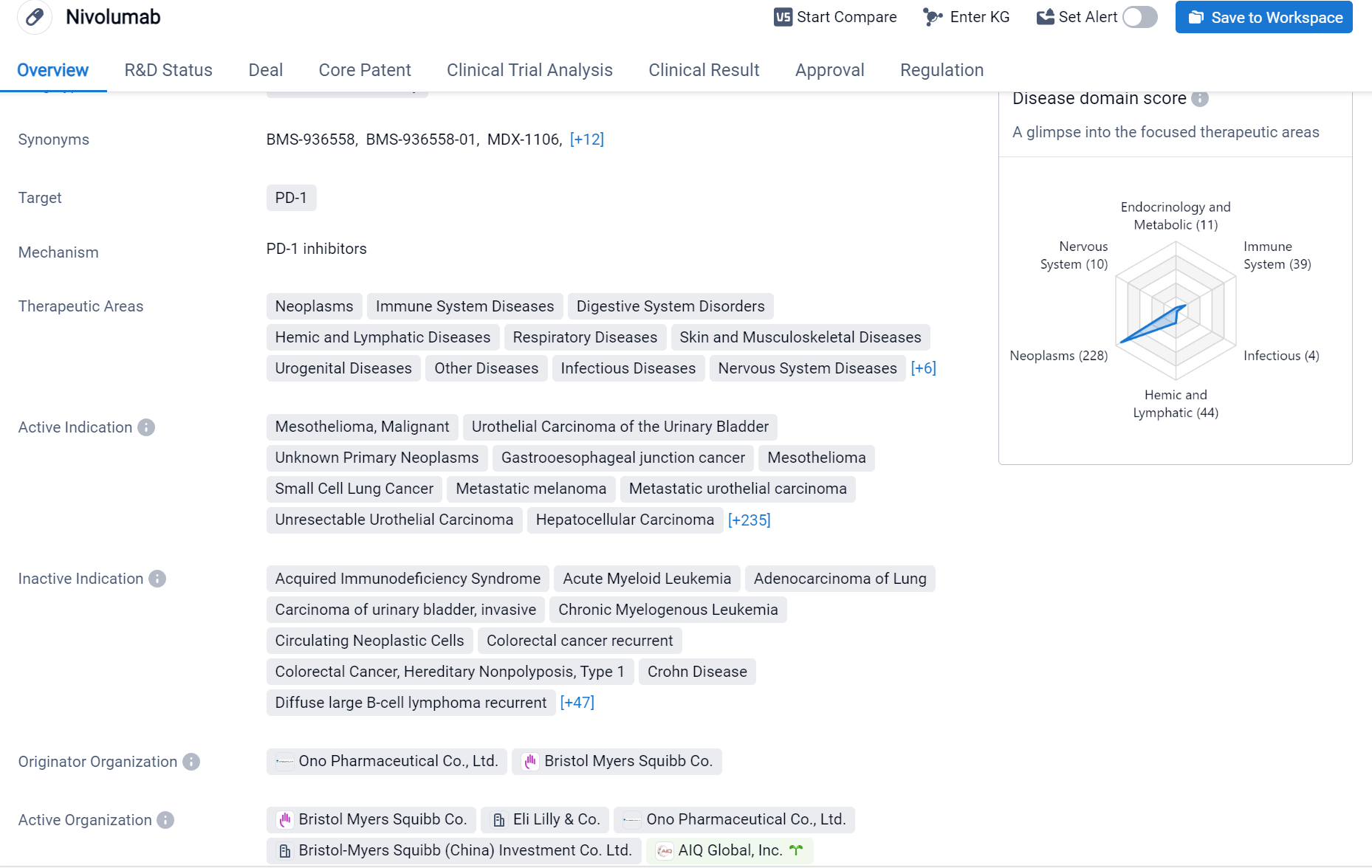
Nivolumab is a monoclonal antibody drug that targets PD-1, a protein found on immune cells. It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, digestive system disorders, hemic and lymphatic diseases, respiratory diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
According to the Patsnap Synapse, Nivolumab was first approved in Japan in July 2014 and has since received approvals in various countries. It is developed by Ono Pharmaceutical Co., Ltd. and Bristol Myers Squibb Co. And the clinical trial distributions for Nivolumab are primarily in the United States, China and United Kingdom. The key indication is Neoplasms.
Detailed Clinical Result of Nivolumab
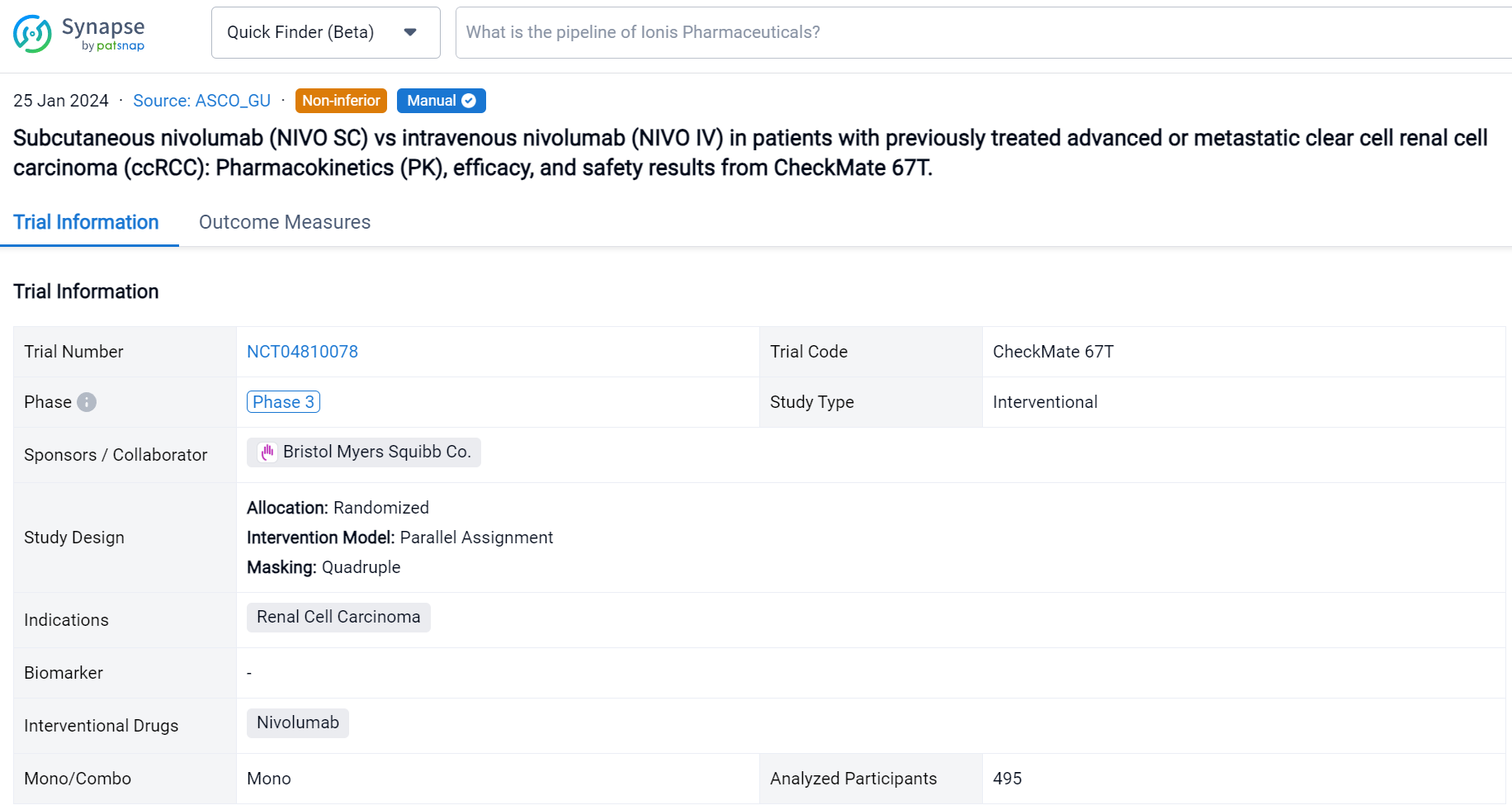
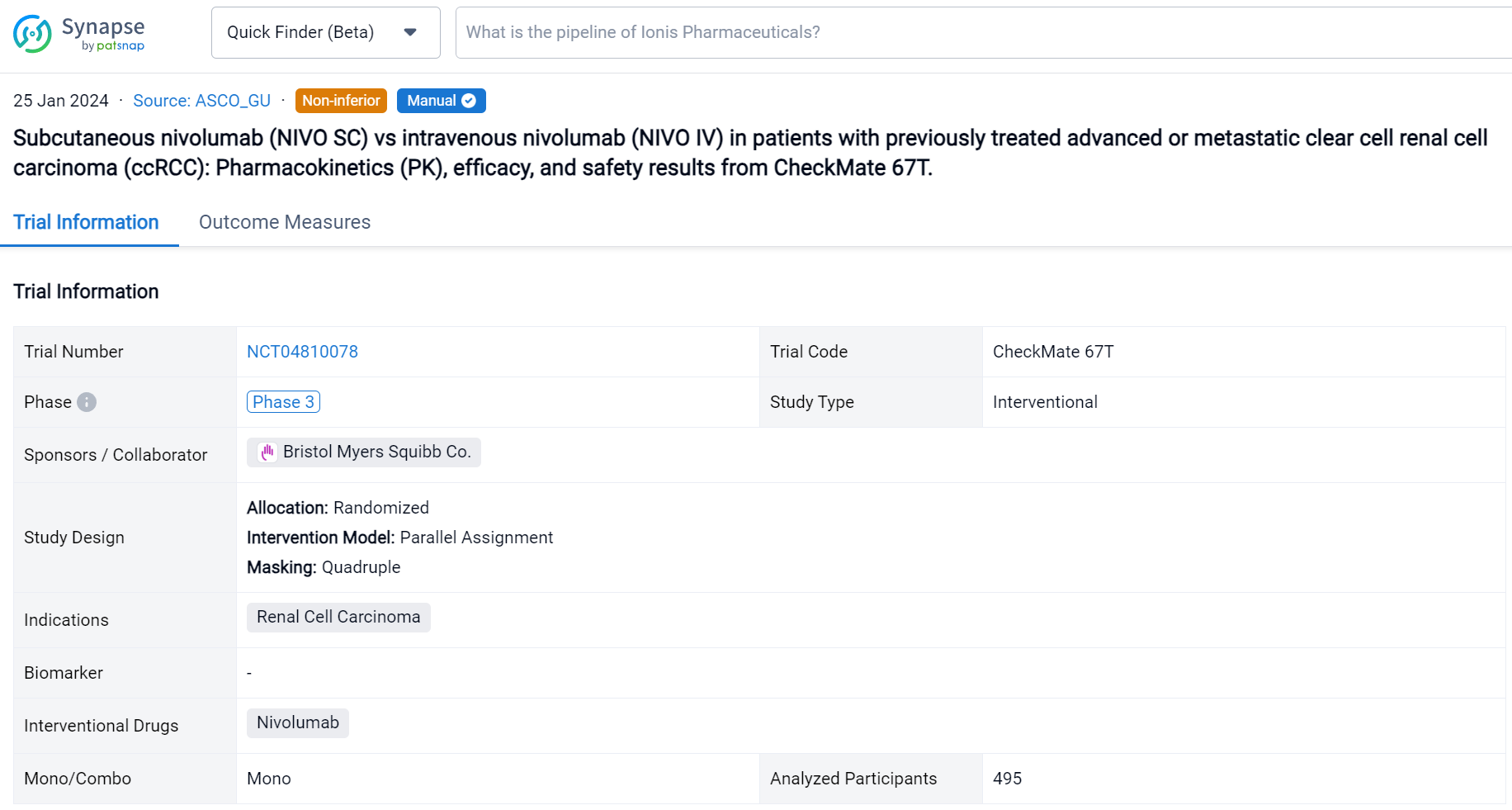
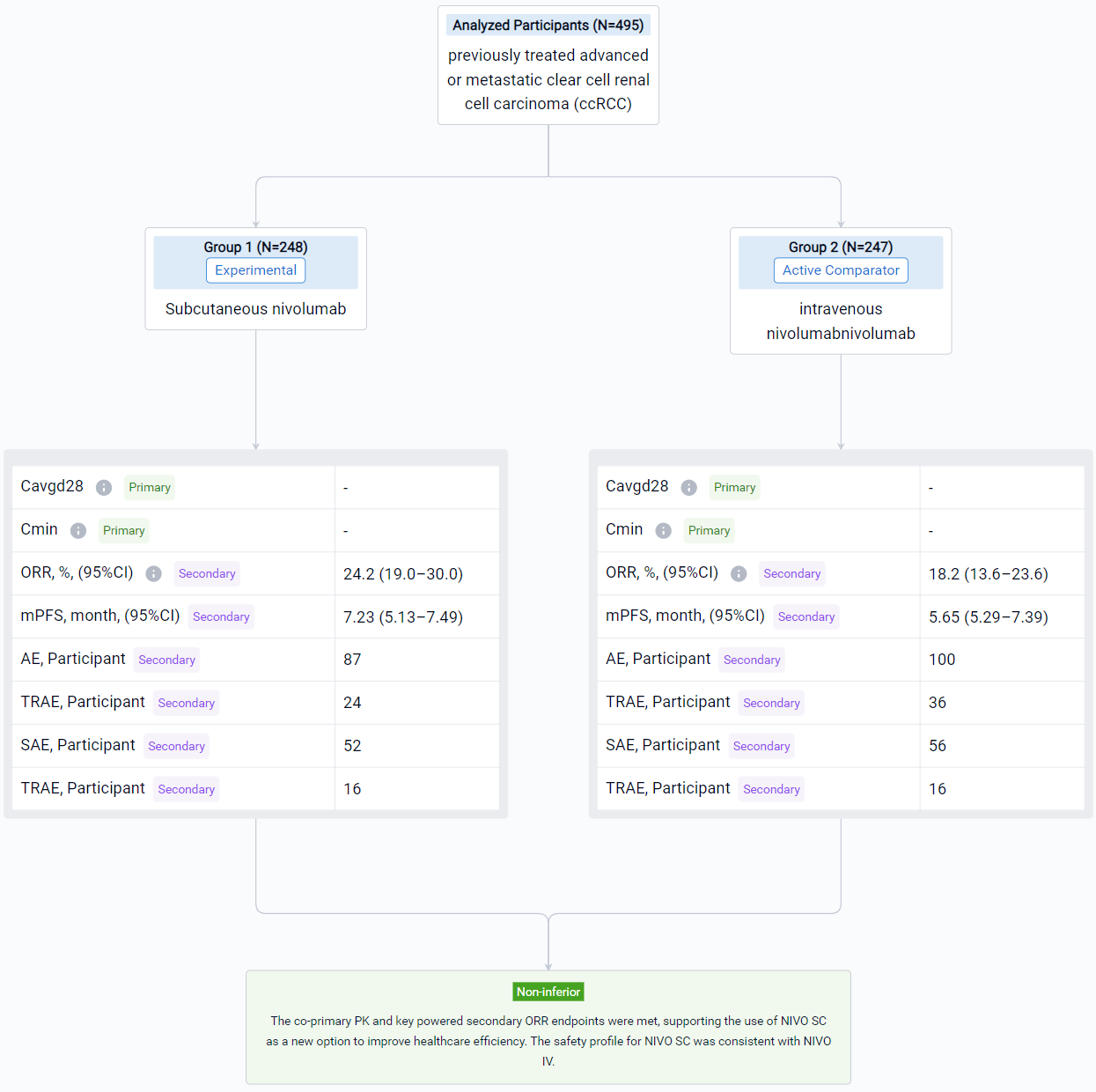
This multicenter, randomized, open-label, phase 3 study (NCT04810078) evaluated PK and objective response rate (ORR) noninferiority of NIVO SC vs IV in patients (pts) with locally advanced or metastatic ccRCC.
In this study, enrolled pts had measurable disease that progressed during or after 1–2 prior systemic regimens, no prior immuno-oncology treatment, and a Karnofsky performance score ≥ 70. Pts were randomized 1:1 to receive NIVO SC 1200 mg + recombinant human hyaluronidase PH20 Q4W or NIVO IV 3 mg/kg Q2W until disease progression, unacceptable toxicity, withdrawal of consent, completion of 2 years’ treatment, or death. The co-primary PK endpoints for noninferiority testing were time-averaged serum concentration over the first 28 days (Cavgd28) and minimum serum concentration at steady state (Cminss) determined by a population PK analysis. ORR by blinded independent central review (BICR) was a key powered secondary endpoint for noninferiority testing. Other secondary objectives included additional PK exposure measures, safety, efficacy, and immunogenicity.
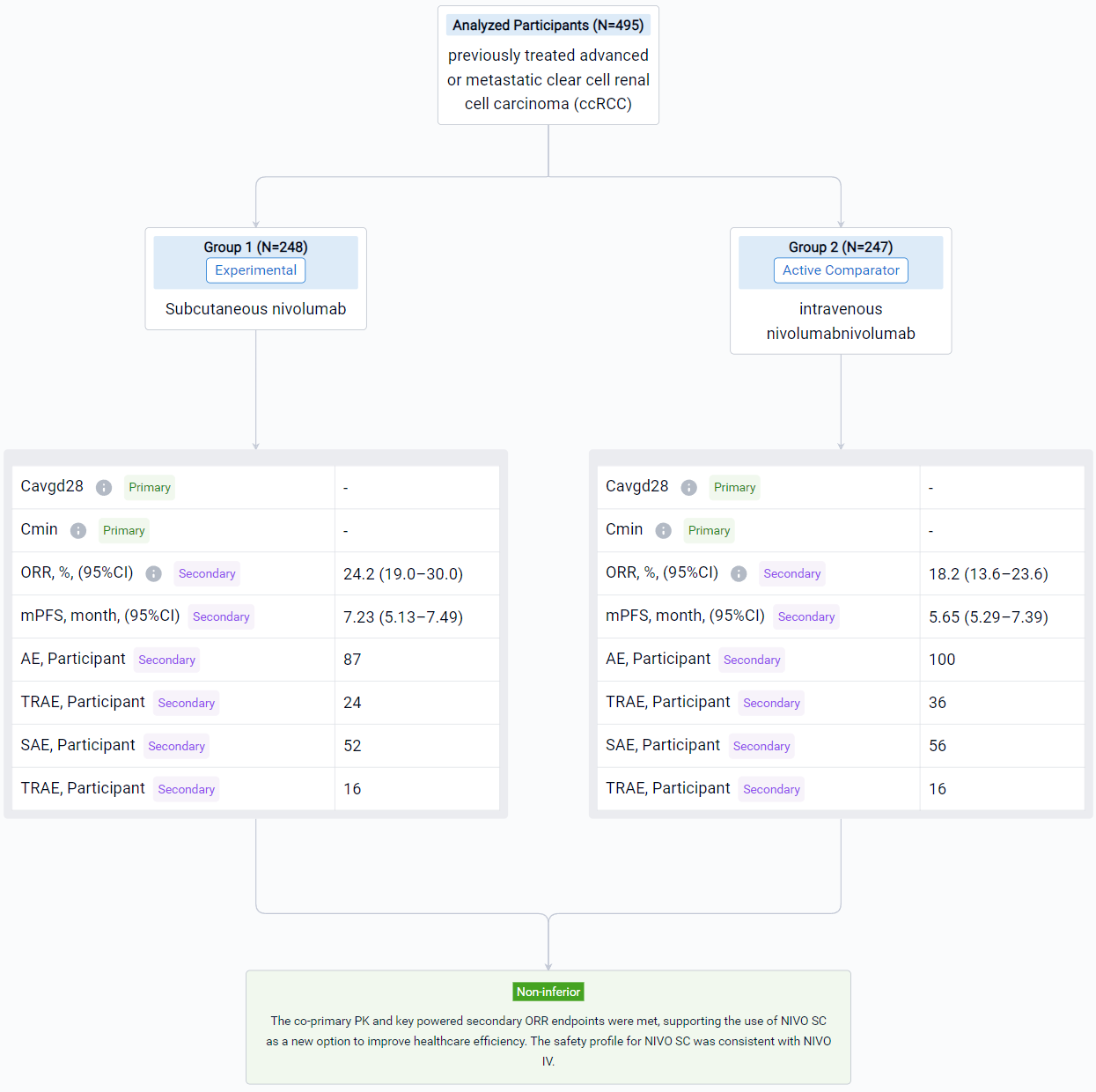
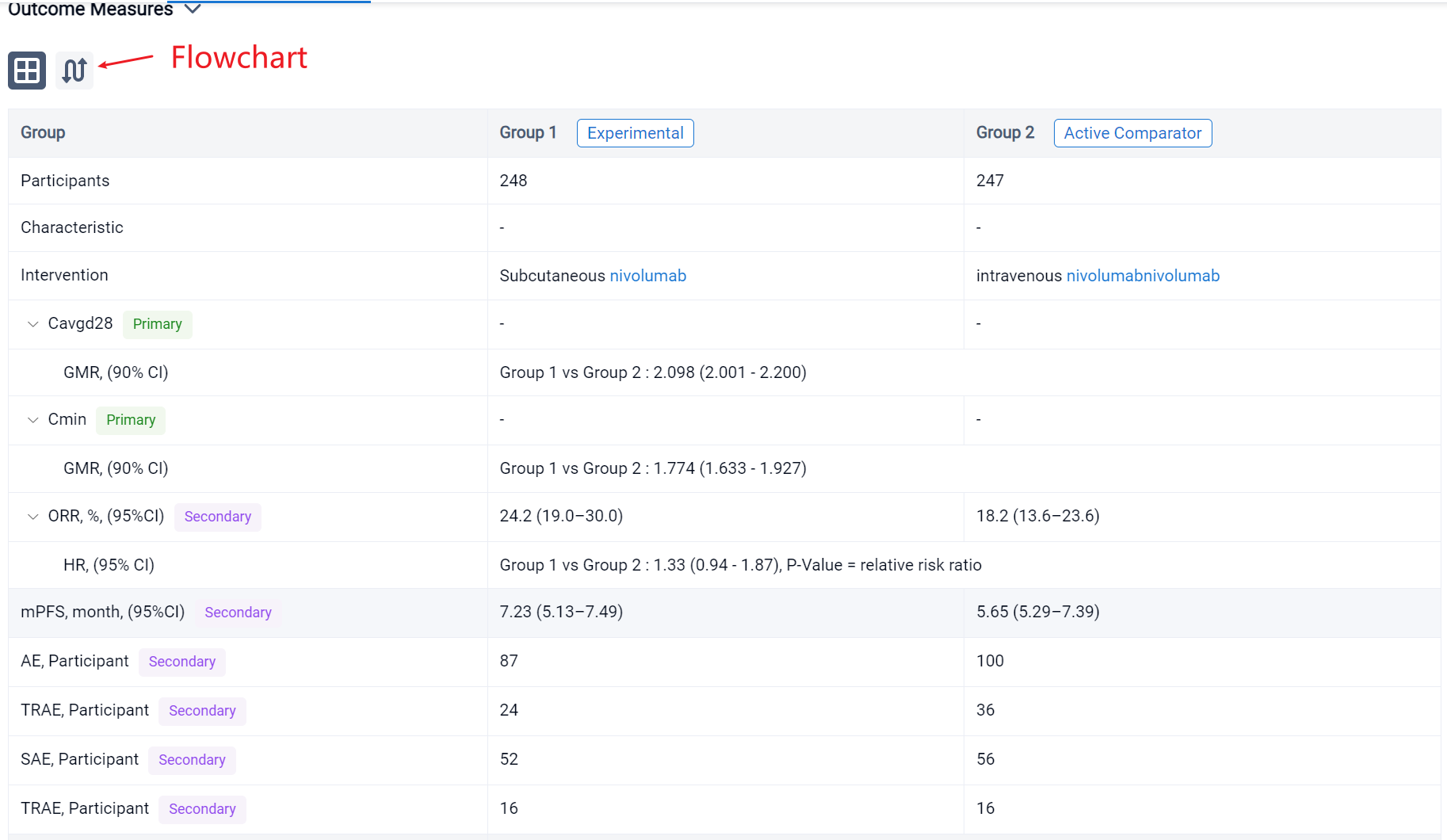
The result showed that a total of 495 pts were randomized to NIVO SC (n = 248) or NIVO IV (n = 247). The median age was 64/66 years in the SC/IV arms. Most pts were male. Average injection time with NIVO SC was < 5 minutes. Noninferiority for the co-primary PK and key powered secondary ORR endpoints was met (see table). Incidence of NIVO SC local injection-site reactions was 8.1%; reactions were low grade and transient. Most deaths were due to disease progression; study drug toxicity led to 3/1 deaths with NIVO SC/IV.
It can be concluded that the co-primary PK and key powered secondary ORR endpoints were met, supporting the use of NIVO SC as a new option to improve healthcare efficiency. The safety profile for NIVO SC was consistent with NIVO IV.
How to Easily View the Clinical Results Using Synapse Database?
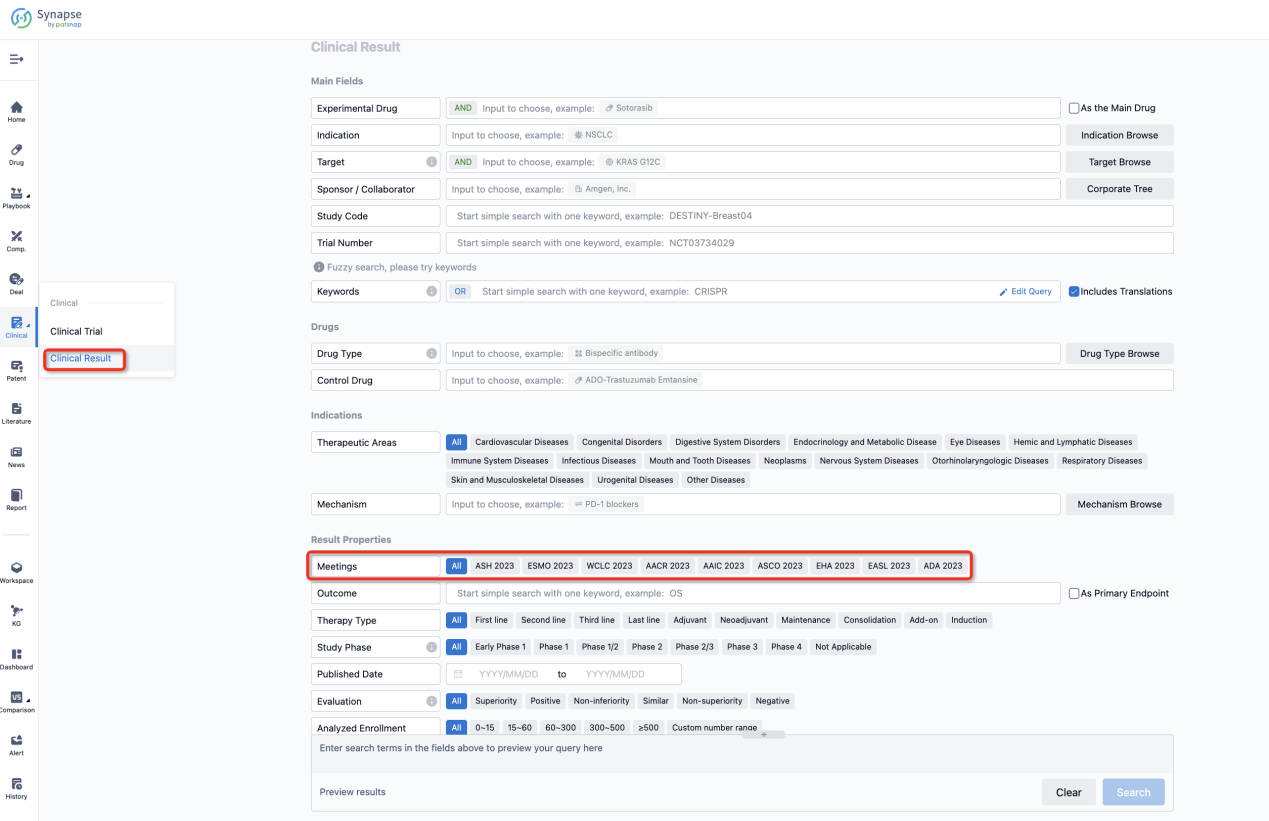
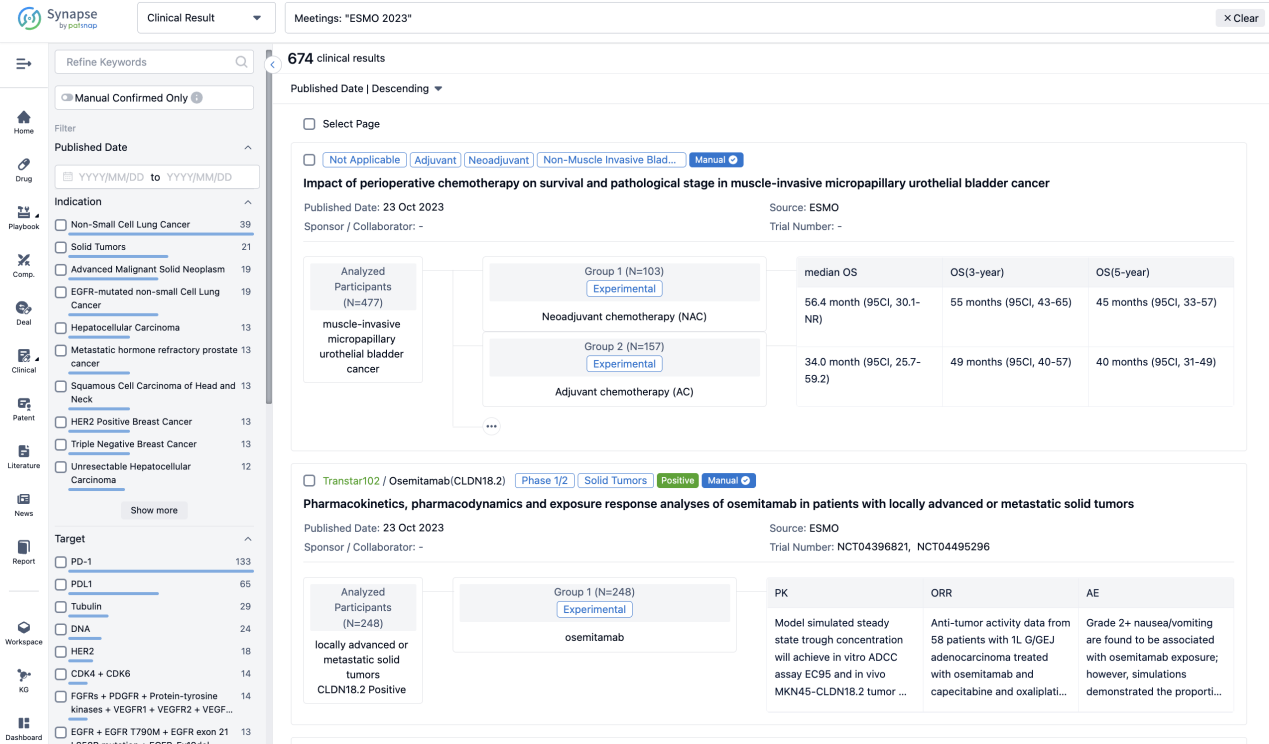
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!