Mabspace Biosciences initiates global Phase III clinical trials for CLDN 18.2 monoclonal antibody TST001
On September 1, 2023, Mabspace Biosciences registered an international multicenter Phase III clinical trial on the Drug Clinical Trial Registration and Information Publicity Platform. The trial aims to evaluate the effectiveness and safety of its CLDN 18.2 monoclonal antibody, TST001, in combination with navulizumab monoclonal antibody and first-line chemotherapy for the treatment of locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma that is Claudin 18.2 positive/HER2 negative.
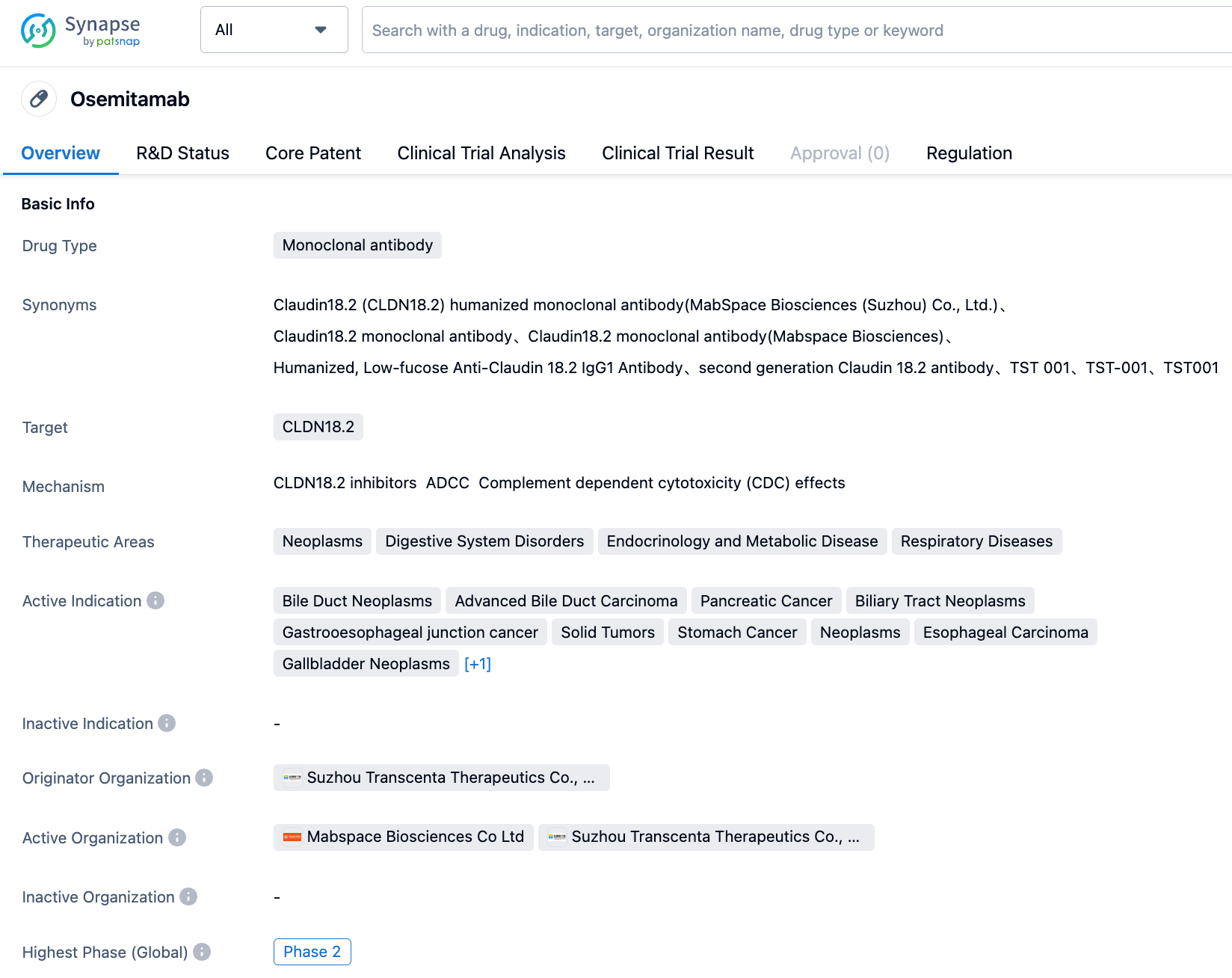
Osemitamab (TST001) is a highly affine, humanized monoclonal antibody targeting Claudin18.2 developed by Mabspace Biosciences. The antibody possesses enhanced Antibody-dependent cellular cytotoxicity (ADCC) and Complement-dependent cytotoxicity (CDC) activity and has demonstrated strong anti-tumour activity in xenotransplantation experiments. Osemitamab (TST001) kills tumor cells expressing Claudin18.2 through ADCC and CDC mechanisms. Leveraging advanced bioprocessing technology, the fucose content of Osemitamab (TST001) is greatly reduced during the production process, thus further enhancing the NK cell-mediated ADCC activity of Osemitamab (TST001).
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Previously, Mabspace Biosciences announced the efficacy data for Osemitamab (TST001) in combination with CAPOX as a first-line treatment for gastric or gastroesophageal junction adenocarcinoma at the ASCO Annual Meeting and the ESMO GI Annual Meeting in 2023. 64 CLDN18.2 positive patients (defined as: CLDN18.2 IHC membranous staining intensity of ≥1+ in ≥10% of tumor cells by the LDT method, screening out about 55% of patients) received treatment, of which 49 received a dose of 6mg/kg. The data shows that in all dose groups, the expected median progression-free survival was 9.5 months, consistent with all levels of CLDN18.2 expression, and the expected median duration of remission was 9.9 months.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
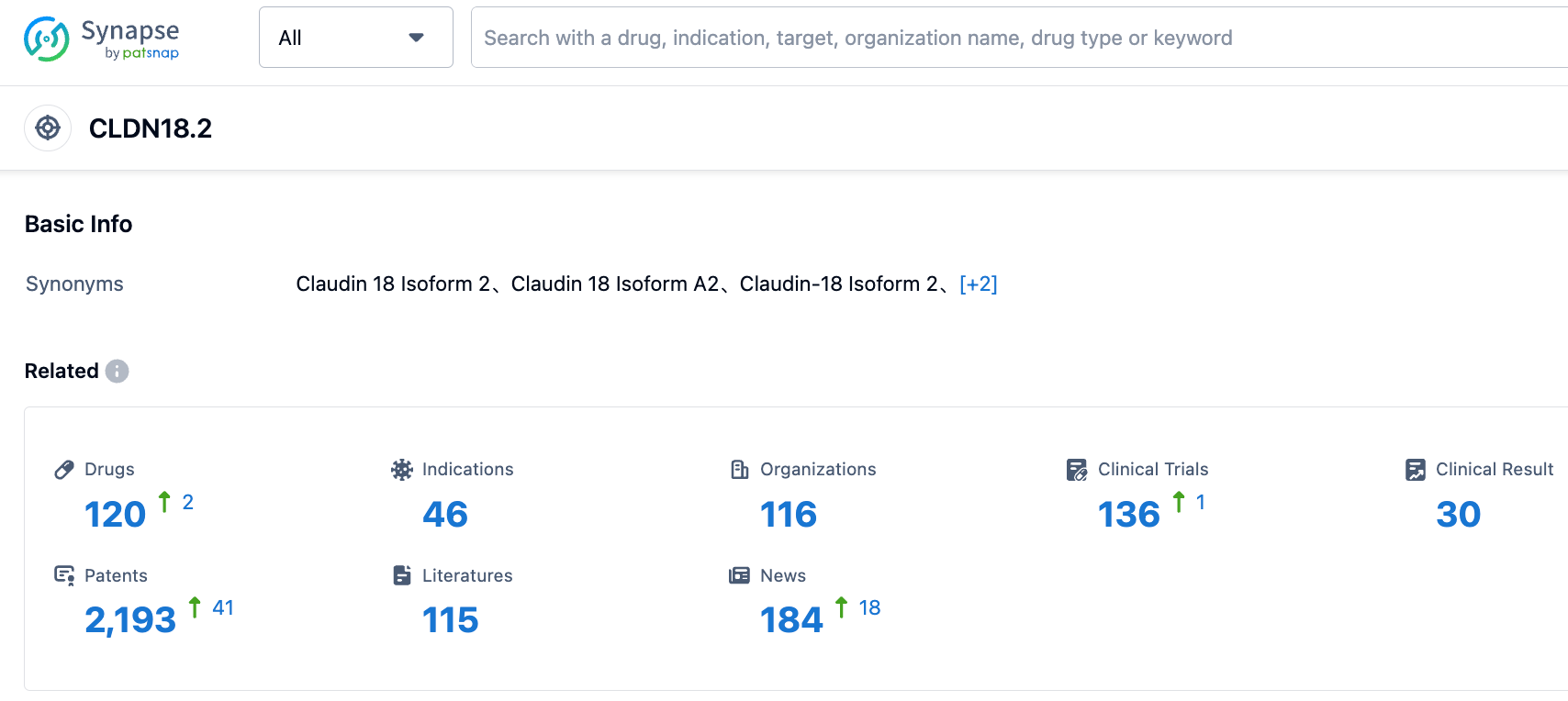
According to the information disclosed by the Synapse database, as of September 2, 2023, there are a total of 120 drugs under research targeting CLDN18.2, covering 46 indications, with 116 research institutions involved, comprising 139 related clinical trials, and as many as 2191 patents. The research and development trend for the CLDN18.2 target is very high. We look forward to the early market introduction of new drugs targeting CLDN18.2.