AstraZeneca's BTK Inhibitor Acalabrutinib gets Approval for New Indications in China
On September 1, 2023, the NMPA officially approved a new indication for AstraZeneca's blood cancer product, Acalabrutinib, for adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who have been previously treated with at least one therapy. Earlier this year, in March, this drug was approved in China for adults with mantle cell lymphoma (MCL) who also had at least one previous treatment.
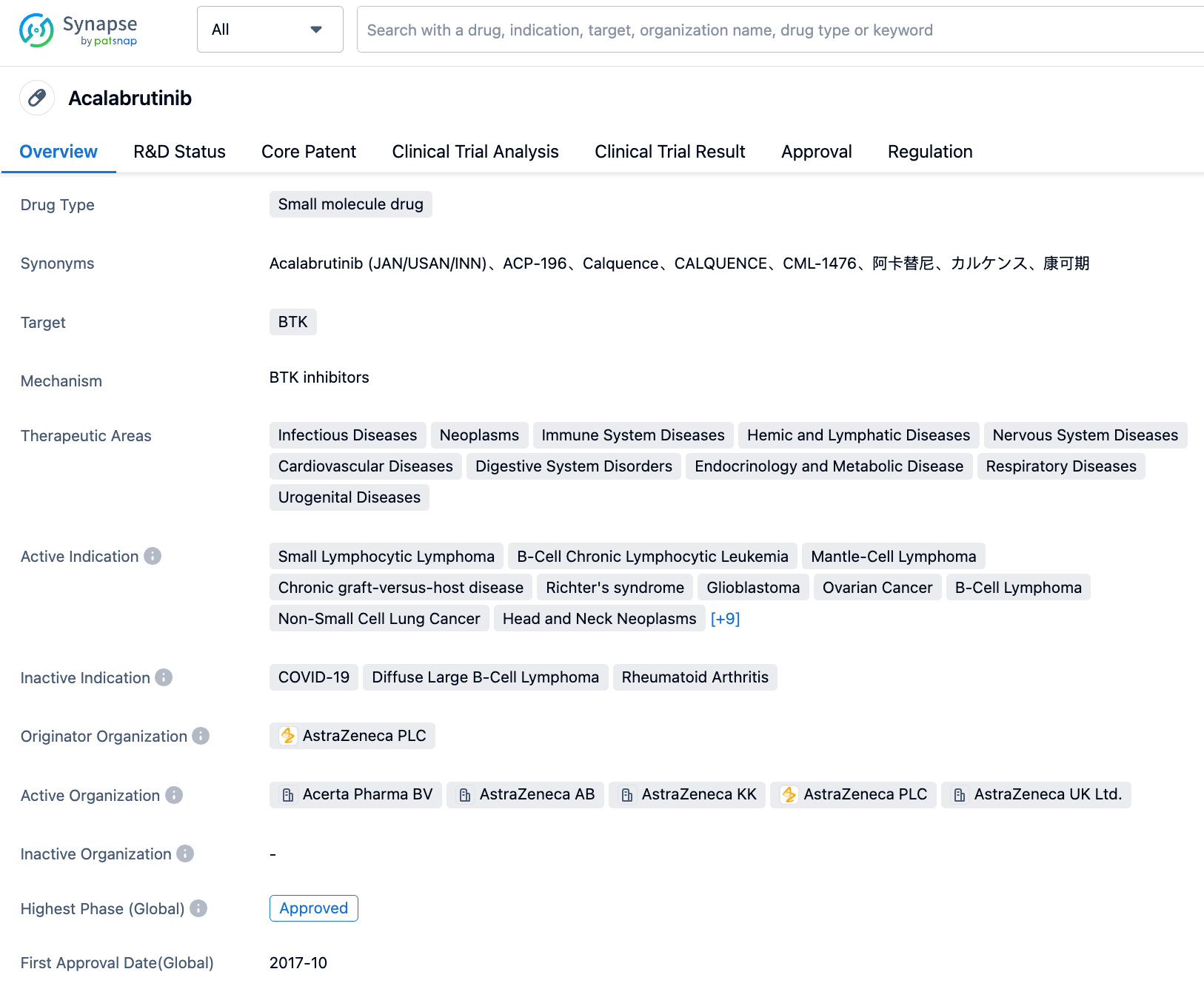
Acalabrutinib is a second-generation selective BTK inhibitor independently developed by AstraZeneca, which inhibits its activity by covalently binding with BTK. In B-cells, BTK signals activate the pathways needed for B-cell proliferation, trafficking, chemotaxis, and adhesion. Preclinical experiments indicate that Acalabrutinib has a high affinity and specificity for BTK. In October 2017, Acalabrutinib was granted accelerated approval by the U.S. FDA for the second-line treatment of MCL. In November 2019, Acalabrutinib was again approved by the FDA to be used as initial or subsequent therapy for adult patients with CLL or SLL. In March 2023, the Acalabrutinib capsule was approved in China for MCL. In December 2022, the second indication for Acalabrutinib was filed for market approval in China, for treating relapsed or refractory CLL.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The approval of the new indication for acalabrutinib is based on the positive results from the global phase 3 ASCEND study and the key phase 1/2 study in China. The ASCEND study explored the efficacy and safety of acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia (CLL), enrolling 310 patients with a median age of 67 years. Based on the data announced by researchers at the 2022 American Society of Clinical Oncology (ASCO) and European Hematology Association (EHA) annual meetings, results from a median follow-up of 46.5 months (approximately 4 years) showed that patients treated with acalabrutinib demonstrated continued progression-free survival (PFS) benefits (based on investigator assessment) with the 42-month PFS and overall survival (OS) rates at 62% and 78%, respectively. The final analysis showed acalabrutinib significantly reduced the risk of death or progression by 72% and had lower adverse event risks, demonstrating sustained long-term safety.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
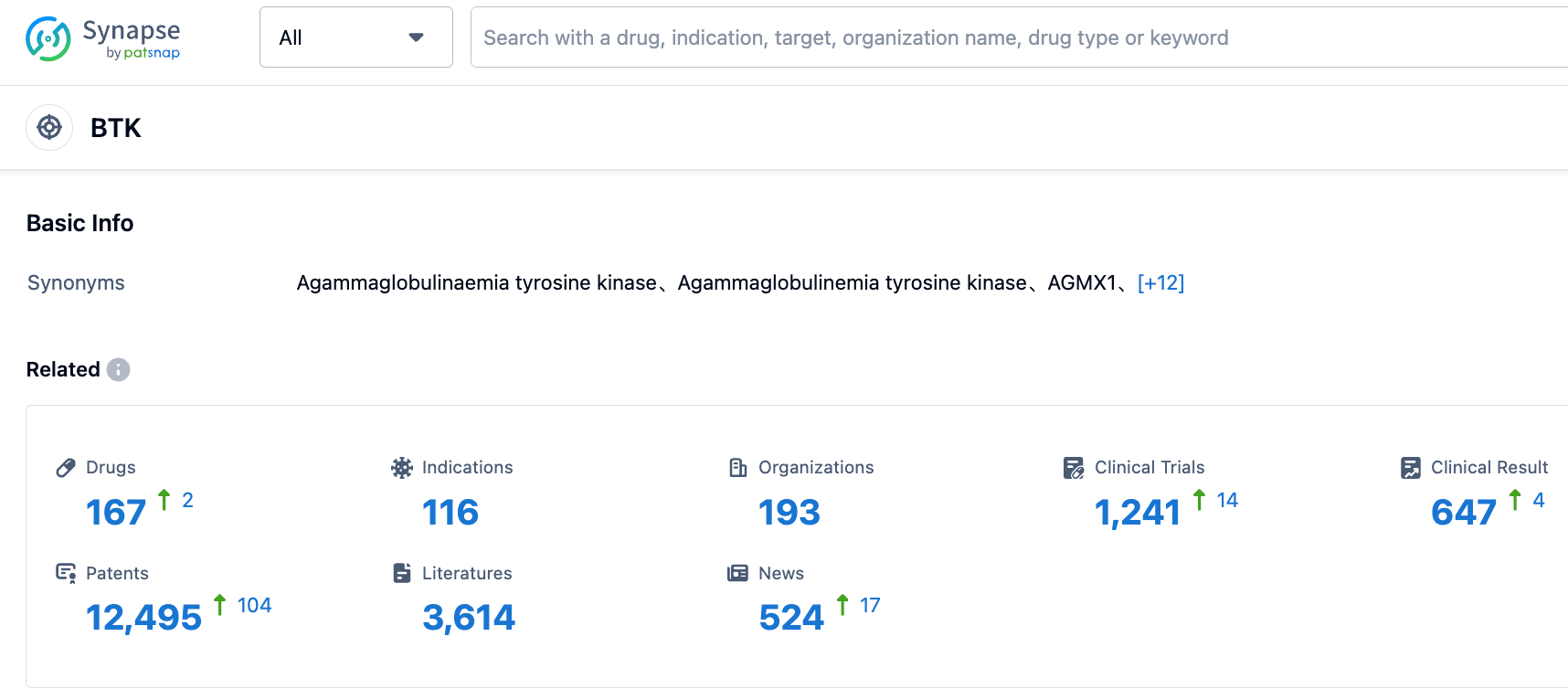
According to the information revealed by the Synapse database, as of September 2, 2023, there are 167 drugs under research targeting BTK, with 116 indications included. There are 193 research institutions involved and 1234 related clinical trials are being conducted. Furthermore, there are as many as 12496 related patents.
Presently, there are already six BTK inhibitors available globally. Besides acalabrutinib, the other five are ibrutinib (AbbVie/Johnson & Johnson), zanubrutinib (BeiGene), orelabrutinib (InnoCare Pharma/Biogen) and tirabrutinib (Ono Pharmaceutical/Gilead Sciences), pirtobrutinib (Eli Lilly, BTK C481S inhibitor). The new indication of acalabrutinib approved in China is expected to boost its market sales.