The FDA has granted IND approval to Biosyngen's BST02, a groundbreaking TIL therapy for liver cancer, the first of its kind globally
The US FDA has given the green light for Biosyngen's BST02 to go forward with clinical studies. This revolutionary TIL therapy for liver cancer, BST02, is a leading product in cellular and gene therapy and signifies the first global TIL treatment aimed at all forms of liver cancer to advance to the clinical phase.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
TIL therapy involves the isolation of natural infiltrating lymphocytes from tumor tissue, followed Within a regulated lab setting, lymphocytes are consummately fortified by undergoing in vitro proliferation to improve their performance. After optimization, these lymphocytes are consequently reintroduced into the patient's body.
TIL therapy presents various commendable benefits, such as being host to several types of TCR clones, increasing tumor-targeting efficiency, and minimizing toxicity. Consequently, TIL therapy holds substantial potential in the management of solid tumors.
Biosyngen's BST02 gaining approval is a landmark achievement for the company. It signifies the fourth innovative product in the company's pipeline that has successfully acquired IND status, leveraging Biosyngen's unique and efficient world-class integrated R&D translational structure.
Over the recent nine months, Biosyngen has been awarded IND approval for four groundbreaking products in both China and the United States, enhancing the company's standing as an ascendant biotech with proprietary R&D expertise, with a focus on CAR-T, TCR-T, and TIL therapies in the T cell therapy discipline.
The adoption of tumor-specific T cells, namely, CAR-T, TCR-T, and TIL therapies, represents a considerable progression in treating solid tumors. Although they share a shared development path, their technology strategies, development, and production processes might differ.
Biosyngen currently boasts comprehensive technological platforms and a databank, including IDENTIFIER®, providing a robust base for the detection and identification of antigens, antibodies, and TCR as well as developing various therapeutic products. Through sustainable and constant diligent efforts, Biosyngen meets the clinical needs that remain unfulfilled, furthering the development of vital, innovative immunotherapy drugs that offer substantial benefits to patients globally.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
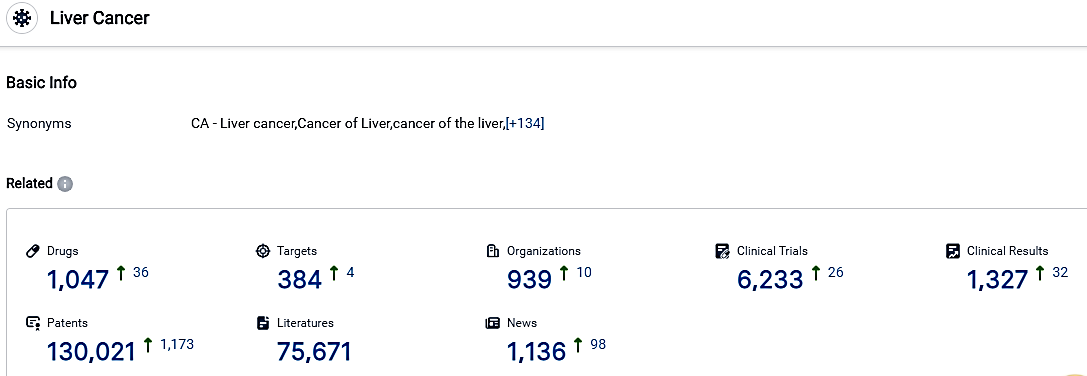
According to the data provided by the Synapse Database, As of October 30, 2023, there are 1047 investigational drugs for the Liver Cancer, including 1047 targets, 939 R&D institutions involved, with related clinical trials reaching 6233,and as many as 130021 patents.
BST02 is an innovative method of adoptive immune cell therapy, which encompasses the enlargement of tumor invading lymphocytes taken from the individual's cells. The treatment is tailored specifically for liver cancer, such as hepatocellular carcinoma and cholangiocarcinoma cases. BST02 has triumphed over identified limitations by utilising cryopreservation, alleviating the issues that arise from geographical distances, and removes the requirement for elevated amounts of interleukin-2. Initial results gathered from preliminary clinical studies have provided proof of its safety and effectiveness.