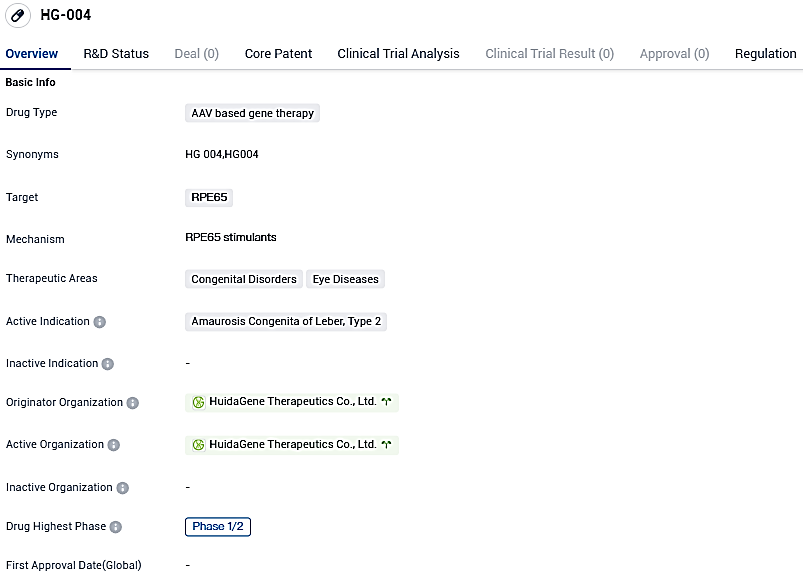
The first participant has been administered HG004 in an international phase 1/2 study against hereditary blindness, as disclosed by Huidagene Therapeutics
HuidaGene Therapeutics, a worldwide oncology-focused biotech firm specializing in the creation of CRISPR-influenced programmable genomic drugs, has declared that the first individual has been treated in the multinational, master-protocol phase 1/2a clinical investigation of its HG004 gene therapy medicine, also known as the STAR study. The drug, a potential gene replacement therapy making use of adeno-associated virus 9, is aimed at addressing inherited retinal dystrophies which are caused by mutations in the RPE65 gene.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"For retinal ailments linked to RPE65, the frequently utilized AAV2 requires a significant overall vector quantity and a large injection amount, which brings up safety worries such as possible retinal detachment," mentioned Alvin Luk, Ph.D., M.B.A., C.C.R.A., Co-founder and top executive officer of HuidaGene. "The demonstration of efficient retinal transduction in targeted RPE layers by HG004 for retinal disorders, allowing a decrease in both overall vector quantity and injection amount, was confirmed in the HG00401 trial for treating children and adults with LCA2."
HG004 represents a gene replacement therapy candidate developed autonomously by HuidaGene. The HG004 treatment employs the man-made AAV9 vector to induce a functioning human RPE65 gene into the retina, with the goal to restore sight and stave off further blindness in children and adults suffering from RPE65-IRDs.
For Leber congenital amaurosis type 2 (LCA2), the HG00401 trial, which employs the identically produced HG004, has been used in a multi-national and multi-site master-protocol trial for RPE65-IRDs. It was recently revealed that the final participant dosage has been delivered, with noticeable sight enhancement and zero safety issues in both adult and juvenile participants.
"This achievement underscores our dedication to bringing groundbreaking genetic treatments to patients with high demand and showcases the effective capability of our team. We desire that this multi-regional trial will offer novel, transformative therapeutic alternatives for individuals around the globe affected by RPE65-IRD," said Xuan Yao, Ph.D., co-founder and regional leader for Greater China at HuidaGene.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
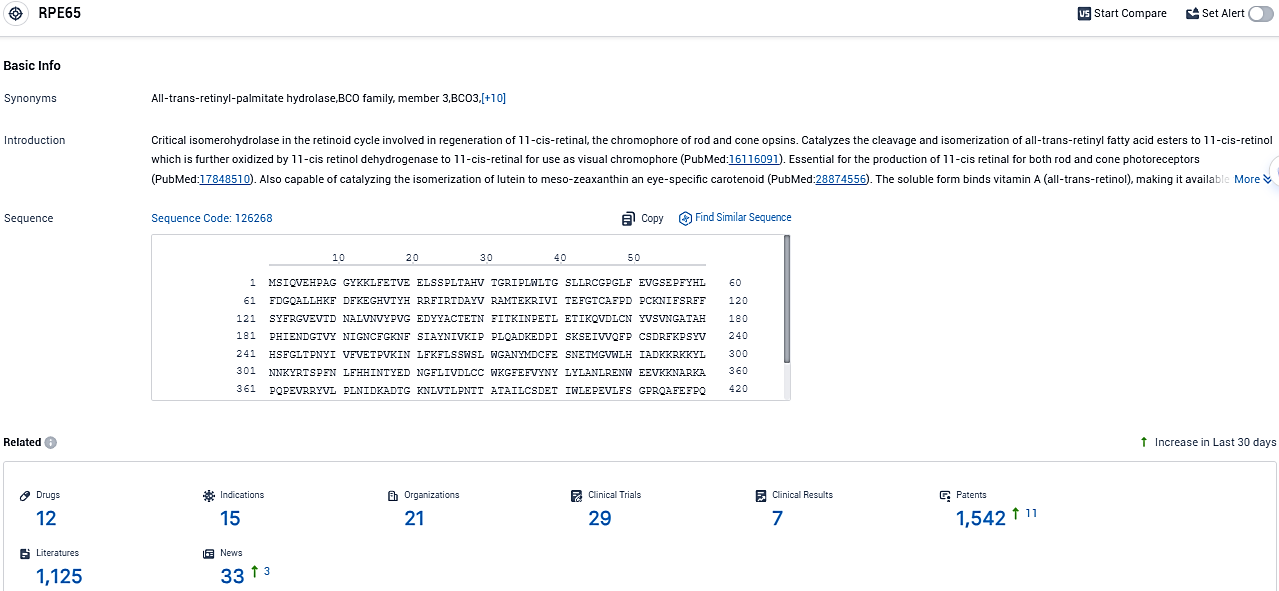
According to the data provided by the Synapse Database, As of November 6, 2023, there are 12 investigational drugs for the RPE65 target, including 15 indications, 21 R&D institutions involved, with related clinical trials reaching 29, and as many as 1125 patents.
This multinational, multicenter, multiple-cohort, dose-finding, and master-protocol clinical trial is designed to evaluate the safety, tolerability, and efficacy of HG004 in patients with inherited retinal dystrophies caused by RPE65 mutations. The primary endpoints include adverse events, certain laboratory measures, and ophthalmic examinations. This HG00402 study will also assess visual function via. multi-luminance mobility test where subjects will be navigating a mobility course under a variety of light levels. Following the completion of the primary study period, subjects will continue to be assessed in a long-term follow-up study of HG004.