The phase 2 clinical trial of Otsuka/Visterra's anti-APRIL monoclonal antibody, sibeprenlimab, for the treatment of IgA nephropathy is published in the NEJM
Recently, Otsuka Pharmaceutical and Visterra announced that the complete results of Phase II clinical trials of sibeprenlimab (VIS649) for the treatment of Immunoglobulin A (IgA) nephropathy have been published in the New England Journal of Medicine (NEJM) and presented at the 2023 American Society of Nephrology (ASN) Kidney Week.
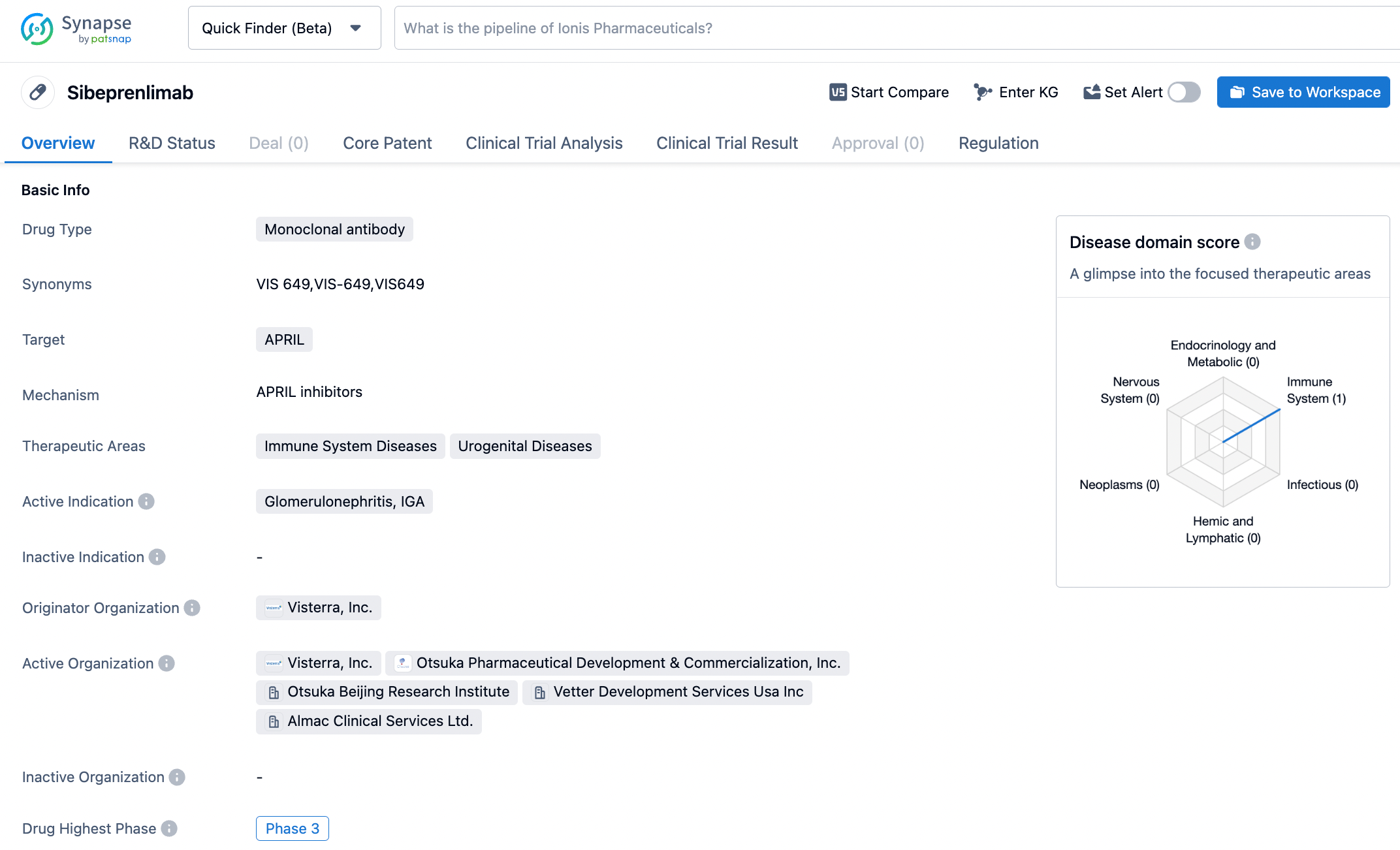
Sibeprenlimab (VIS649) is an antibody against a proliferation-inducing ligand (APRIL). APRIL is a cell factor that stimulates the abnormal production of IgA, and VIS649 has been shown to bind and block the function of APRIL, which may help treat IgA nephropathy. The drug was initially developed by Visterra. In 2018, Otsuka Pharmaceutical announced its acquisition of Visterra for approximately $430 million, thus obtaining a range of products under development for conditions including IgA nephropathy and other kidney diseases, including VIS649. VIS649 is not yet available in any country or region. Otsuka is conducting an international multicenter Phase III clinical trial to evaluate the efficacy and safety of the drug in patients with IgA.
The study results show that after 12 months of treatment with sibeprenlimab, patients with IgA nephropathy had a significant reduction in proteinuria compared with placebo treatment. At the 12-month mark, compared to baseline, the geometric mean ratio of the 24-hour urine protein/creatinine ratio (UPCR) in patients receiving 2, 4, and 8 mg/kg doses of sibeprenlimab and placebo decreased by 47.2%, 58.8%, 62.0%, and 20.0%, respectively. Compared to the placebo group, the estimated glomerular filtration rate (eGFR) also showed beneficial changes in the sibeprenlimab group. The annual changes in eGFR for patients receiving 2, 4 and 8 mg/kg sibeprenlimab and placebo were -2.7, +0.2, -1.5, and -7.4 ml/min/1.73m2, respectively. This indicates that patient's eGFR tended to stabilize after using sibeprenlimab, on contrary, eGFR declined after using placebo. Among patients treated with sibeprenlimab and placebo, the incidence of adverse events related to treatment (TEAE) was similar. During the trial and the subsequent follow-up period till the 16th month, there was no evidence of adverse toxicities or clinically significant immunosuppression in the safety assessment of sibeprenlimab.
According to information disclosed by the Synapse database, as of November 7, 2023, there are 11 drugs under development targeting APRIL, with 25 indications, 24 research institutions involved, 104 related clinical trials, and up to 31,654 patents. Among the drugs under development targeting APRIL, besides Otsuka's VIS649, Chinook Therapeutics' BION-1301, Yakon Bio's APRIL CAR-T therapy, and Alpine Immune Sciences' APRIL/BAFF dual antibody have also entered the clinical research stage. We look forward to subsequent developments in this target track.