Tirzepatide Phase 3 Success for Obese Adults with HFpEF: Lilly's Update
Eli Lilly and Company revealed today the encouraging primary outcomes from the SUMMIT phase 3 trial, which assessed the safety and effectiveness of tirzepatide injection in adults suffering from heart failure with preserved ejection fraction and obesity.
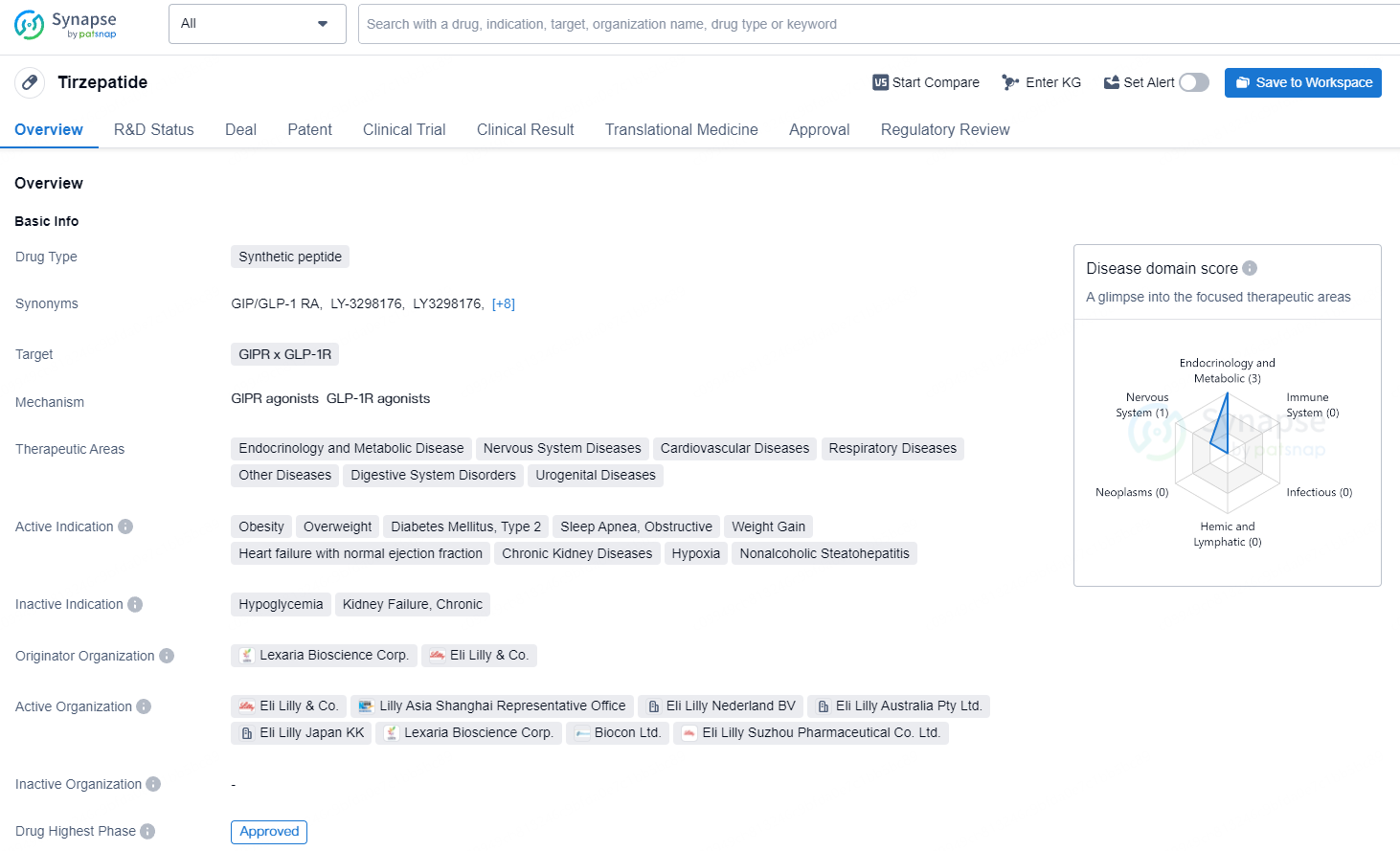
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
In clinical trials, tirzepatide showed significant statistical improvements in the primary endpoints, including a decreased risk of heart failure outcomes measured as a composite endpoint, and enhanced heart failure symptoms and physical limitations based on the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score, when compared to a placebo.
Furthermore, all important secondary endpoints were achieved. These included better exercise capacity as assessed by the 6-Minute Walk-Test Distance, a reduction in the inflammation marker high-sensitivity C-reactive protein, and average body weight reduction from the baseline at 52 weeks.
For the efficacy estimand, tirzepatide resulted in a 15.7% reduction in body weight versus a 2.2% reduction with placebo. For the treatment-regimen estimand, the body weight reduction for tirzepatide was 13.9% compared to 2.2% for placebo.
"HFpEF accounts for nearly half of all heart failure cases, with almost 60% of affected individuals in the U.S. also experiencing obesity. Despite the growing number of people with both HFpEF and obesity, treatment options are still quite limited," stated Jeff Emmick, MD, PhD, senior vice president of product development at Lilly. "Earlier incretin research in this demographic focused on symptomatic and physical limitations. However, this groundbreaking trial with tirzepatide has shown a reduction in symptom severity and improved heart failure outcomes for individuals with HFpEF and obesity."
Lilly will continue analyzing the SUMMIT study results, which will be shared at an upcoming medical conference and submitted to a peer-reviewed journal. They also plan to present the SUMMIT study findings to the U.S. Food and Drug Administration and other regulatory bodies starting later this year.
Tirzepatide, a once-weekly GIP (glucose-dependent insulinotropic polypeptide) receptor and GLP-1 (glucagon-like peptide-1) receptor agonist, is a singular molecule that activates the body's receptors for these natural incretin hormones. These receptors are located in key brain regions involved in appetite control. Tirzepatide has been found to reduce food consumption and influence fat metabolism. Ongoing studies are examining tirzepatide's effects on chronic kidney disease and obesity-related morbidity/mortality. Earlier this year, Lilly also submitted data for tirzepatide in moderate-to-severe obstructive sleep apnea and obesity to the U.S. Food and Drug Administration and other international regulatory authorities.
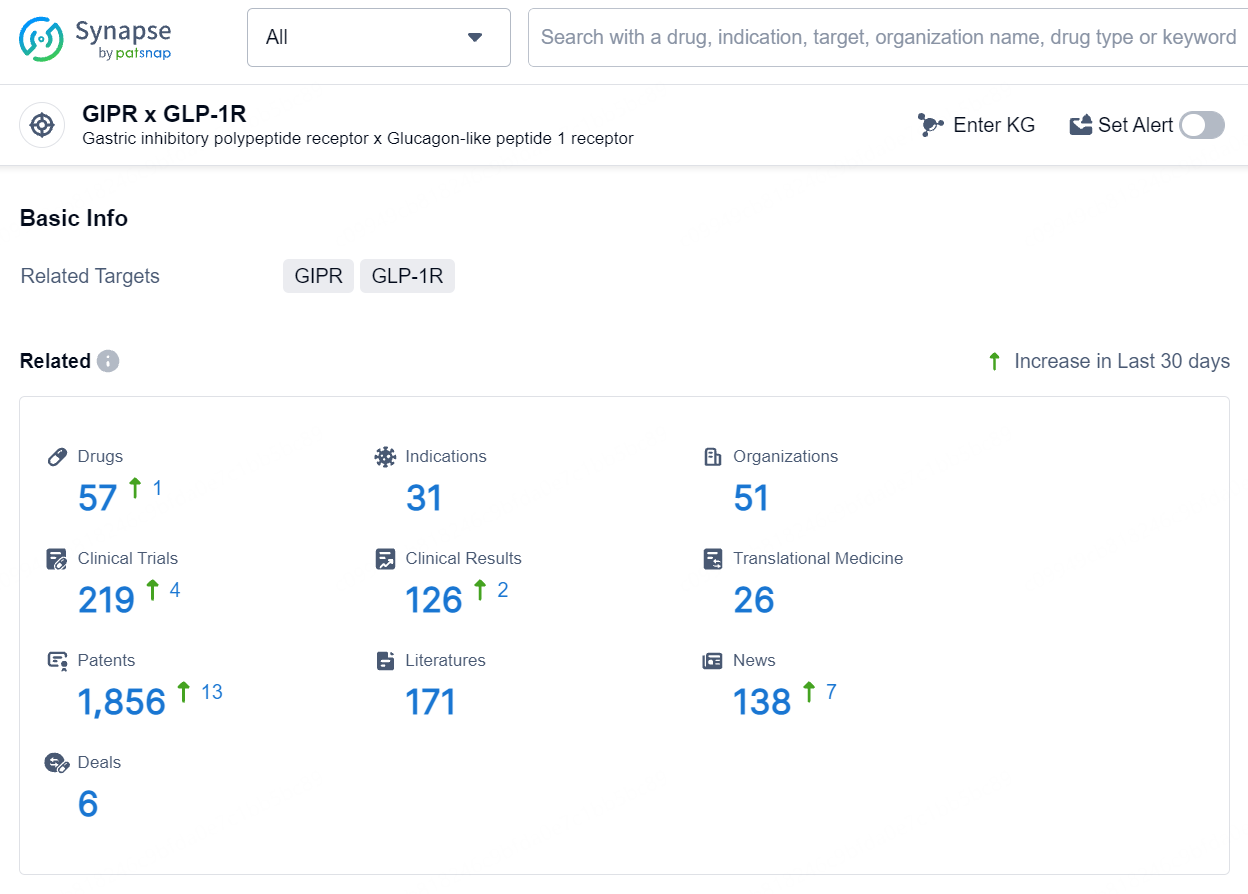
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 6, 2024, there are 57 investigational drugs for the GIP and GLP-1 targets, including 31 indications, 51 R&D institutions involved, with related clinical trials reaching 219, and as many as 1856 patents.
Tirzepatide's approval and its wide-ranging therapeutic areas and active indications underscore its potential to impact the pharmaceutical landscape significantly. Its development and approval reflect the ongoing efforts in the pharmaceutical industry to address complex and prevalent diseases such as obesity, diabetes, and cardiovascular conditions.