TOPAZ-1 Study: Doubling 3-Year Survival with Imfinzi-Chemo Combo in Advanced Biliary Cancer
Recent findings from the TOPAZ-1 Phase III study indicate that when used together with standard chemotherapy, AstraZeneca's Imfinzi (durvalumab) yields a significant long-term survival advantage at three years for individuals suffering from advanced biliary tract cancer.
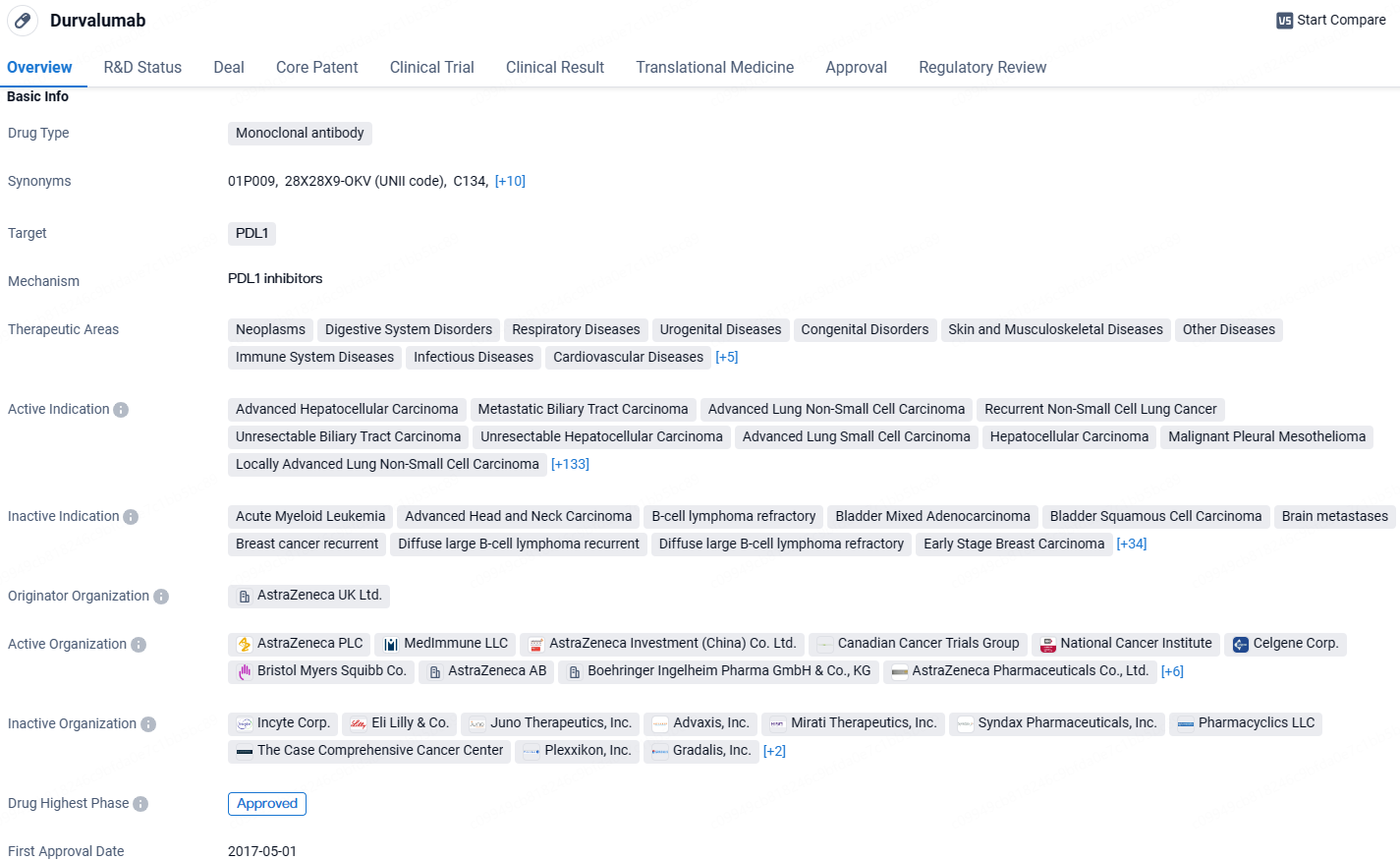
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The findings from the TOPAZ-1 trial, representing the most extended survival monitoring in a worldwide randomized Phase III trial for this type of analysis, will be revealed on April 18 at the 2024 Cholangiocarcinoma Foundation Conference in Salt Lake City, Utah.
The trial showed that after more than three years, Imfinzi combined with chemotherapy dropped the mortality risk by 26% when compared to chemotherapy alone. The median overall survival was 12.9 months for the Imfinzi plus chemotherapy group as opposed to 11.3 months for those receiving only chemotherapy. Additionally, the survival rate at three years was more than double for patients treated with the Imfinzi regimen in contrast to those on chemotherapy alone.
Do-Youn Oh, MD, PhD, a Professor in the Division of Medical Oncology at the Department of Internal Medicine at Seoul National University Hospital and College of Medicine, and the lead researcher for this study, remarked: “The recent TOPAZ-1 data demonstrate a doubling in survival at three years for patients suffering from advanced biliary tract cancer treated with durvalumab and chemotherapy. This progress is particularly significant in a medical context traditionally associated with dismal prognoses. It underscores the enduring value of this treatment combination as a core therapy for this severe condition.”
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, commented: "TOPAZ-1 has significantly elevated treatment standards for advanced biliary tract cancer by demonstrating substantial survival benefits when Imfinzi is included with chemotherapy, in a regimen that is well-received. These findings mark the most prolonged survival follow-up ever documented with an immunotherapy-based approach in this domain. Furthermore, the improvements seen at the three-year milestone highlight our dedication to enhancing survival rates for gastrointestinal cancer patients over the long term.”
Stacie Lindsey, CEO of the Cholangiocarcinoma Foundation, stated: “The extended survival data from AstraZeneca in advanced biliary tract cancer sets a significant benchmark, giving us the first glance at three-year survival rates for these patients. This encourages optimism that ongoing research might further advance patient outcomes in these severe and uncommon forms of cancer.”
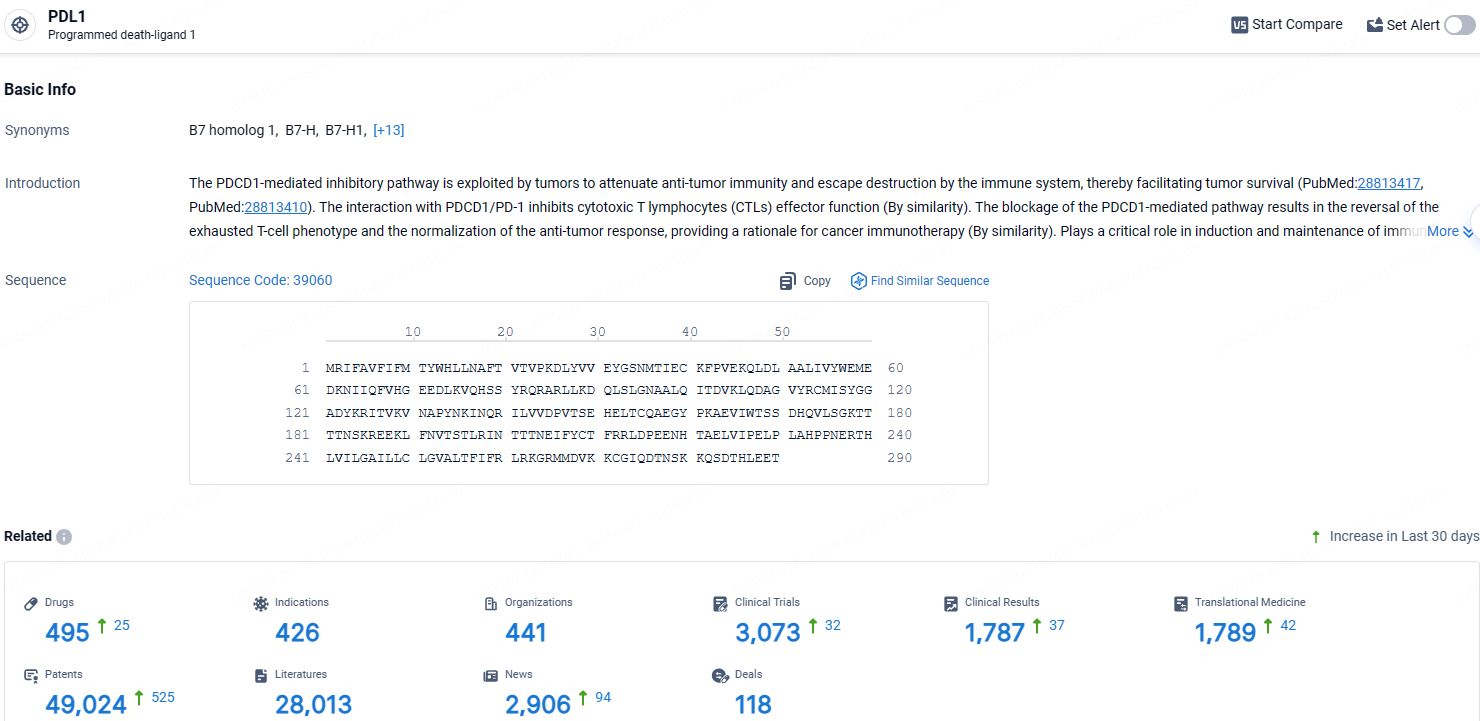
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 18, 2024, there are 495 investigational drugs for the PDL1 target, including 426 indications, 441 R&D institutions involved, with related clinical trials reaching 3073, and as many as 1789 patents.
As a monoclonal antibody targeting PDL1, Durvalumab works by blocking the interaction between PDL1 and PD1, which helps the immune system recognize and attack cancer cells. Durvalumab is a promising drug in the field of biomedicine, with its approval for multiple indications and its potential to improve patient outcomes in various types of cancers. Its mechanism of action and designations highlight its importance in the pharmaceutical industry and its potential to contribute to the treatment of numerous diseases.