TTerns Pharmaceuticals announced first participant treatment in Phase 1 Trials of potential obesity drug TERN-601 Oral GLP-1 Receptor Agonist
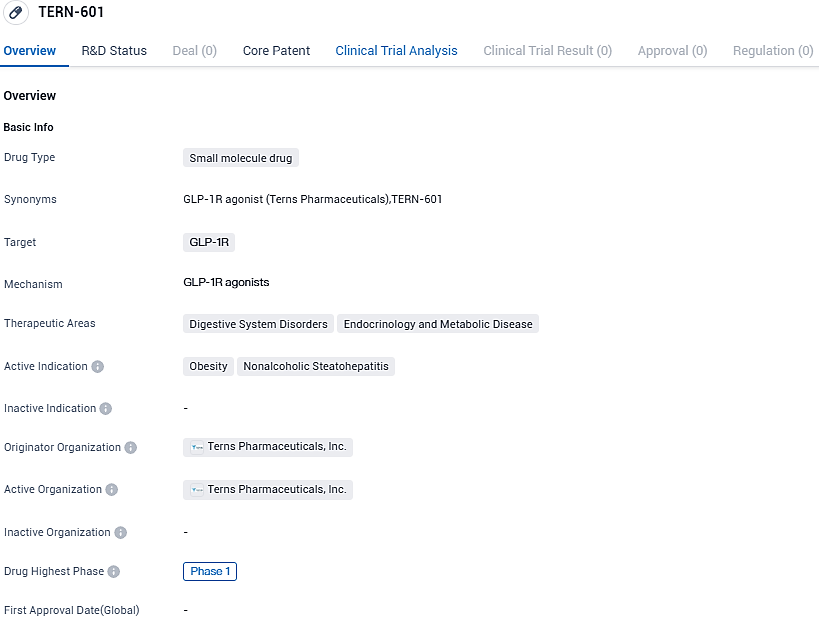
Terns Pharmaceuticals, Inc., a firm in the clinical phase that focuses on creating a range of small-molecule drug contenders to combat severe ailments, such as cancer, obesity, and non-alcoholic steatohepatitis, has revealed that the initial participant has received dosage in the Phase 1 clinical examination of TERN-601. It's the company's orally-administered small-molecule GLP-1R catalyst used in obesity management.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"We are thrilled to commence our premier human trial of the oral GLP-1R agonist, TERN-601, as we feel it could offer an impactful alternative to the current injectable GLP-1R agonist treatments available," commented Erin Quirk, MD, the president and the research and development chief at Terns. She further stated, "TERN-601 is our discovery - the first internal small molecule GLP-1R agonist designed to be taken orally once a day.
It has a competitive edge in weight loss, both as a standalone treatment and as potentially a component of a future all-oral combination therapy for obesity." Terns' prime GLP-1R agonist, TERN-601, was conceived through in-house structure-centric drug discovery maneuvers using our proprietary 3D QSAR model, implemented to pinpoint new GLP-1R agonist contenders. Subsequent optimization of the ligands was then carried out based on in vitro behavior, metabolic steadiness, and pharmacokinetic properties.
TERN-600 and TERN-800 series comprising of additional small molecule GLP-1R agonists and small-molecule glucose-dependent insulinotropic polypeptide receptor manipulators, with the ability to be coupled with GLP-1R agonists.
Alongside TERN-501, a highly selective THR-β agonist under development for NASH treatment, these programs aim to significantly enhance clinical results for patients fighting metabolic diseases, topping the current treatments in terms of potential tolerability, access, and ease of use.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
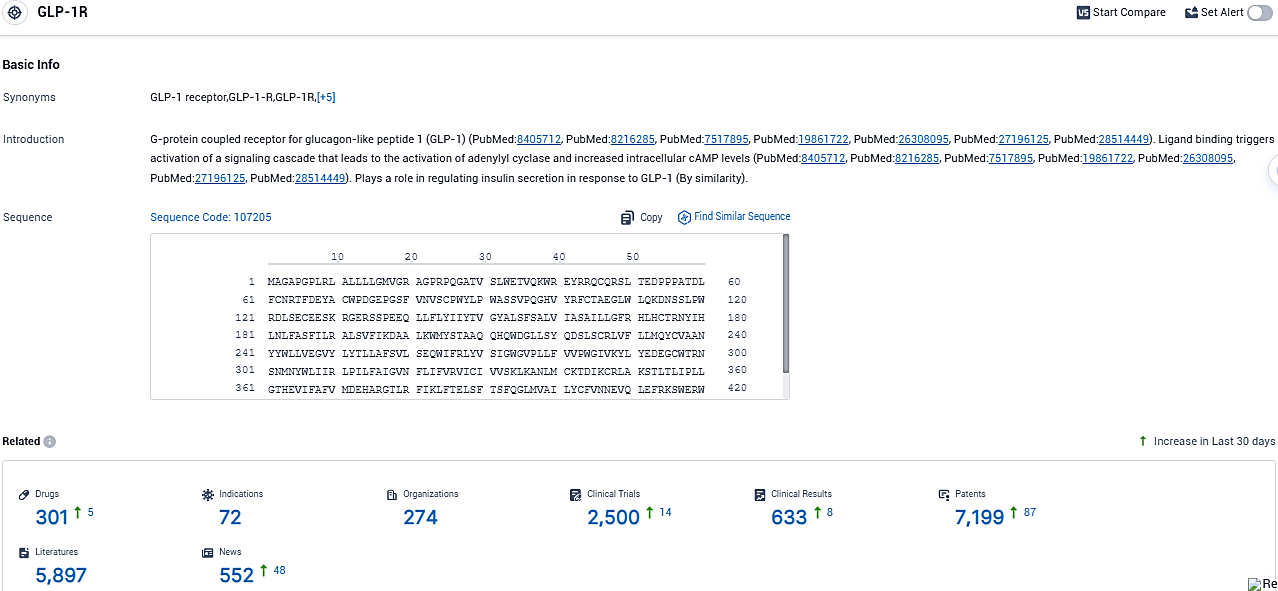
According to the data provided by the Synapse Database, As of November 9, 2023, there are 301 investigational drugs for the GLP-1R target, including 72 indications, 274 R&D institutions involved, with related clinical trials reaching 2500, and as many as 5897 patents.
TERN-601 is an oral, small-molecule glucagon-like peptide-1 receptor, or GLP-1R, agonist program for obesity. Obesity is a chronic disease that is increasing in prevalence in adults, adolescents and children and is often defined by having an elevated BMI of 30 or greater. GLP-1 agonism offers multiple benefits including improved glucose control, slowing of gastric emptying and increases in satiety.