Unleashing the Power of Sevelamer Hydrochloride: A Comprehensive Review on R&D Breakthroughs
Sevelamer Hydrochloride's R&D Progress
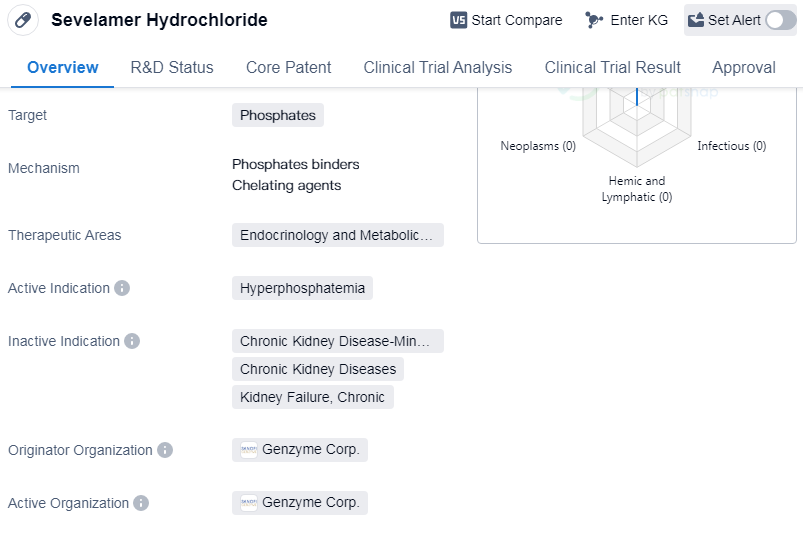
Sevelamer Hydrochloride is a small molecule drug that is primarily used to target phosphates in the body. It falls under the therapeutic areas of endocrinology and metabolic disease, specifically for the treatment of hyperphosphatemia. The drug was first approved in the United States in October 1998.
Sevelamer Hydrochloride is developed by Genzyme Corp., an originator organization in the pharmaceutical industry. It is important to note that the drug has reached the highest phase of development, which is the approved stage. This indicates that it has successfully undergone rigorous testing and evaluation to demonstrate its safety and efficacy.
Hyperphosphatemia is a condition characterized by abnormally high levels of phosphate in the blood. It is commonly associated with chronic kidney disease and can lead to complications such as cardiovascular disease and bone disorders. Sevelamer Hydrochloride works by binding to phosphate in the gastrointestinal tract, preventing its absorption into the bloodstream. This helps to lower phosphate levels and manage the condition.
The approval of Sevelamer Hydrochloride in 1998 marked an important milestone in the treatment of hyperphosphatemia. It provided healthcare professionals with a targeted therapy to address the underlying cause of the condition. Since then, the drug has been widely used in clinical practice and has demonstrated its effectiveness in managing hyperphosphatemia in patients with chronic kidney disease.
The approval of Sevelamer Hydrochloride in the United States also paved the way for its global availability.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Sevelamer Hydrochloride: Phosphate binders and Chelating agents
Phosphate binders are medications or substances that are used to reduce the levels of phosphate in the body. Phosphate is a mineral that is normally found in the body and is important for various biological processes. However, in certain medical conditions such as chronic kidney disease, the body may not be able to effectively eliminate excess phosphate, leading to high levels of phosphate in the blood.
Phosphate binders work by binding to the phosphate in the gastrointestinal tract, preventing its absorption into the bloodstream. This helps to lower the levels of phosphate in the body and prevent complications associated with high phosphate levels, such as bone and mineral disorders.
Chelating agents, on the other hand, are substances that have the ability to bind to metals and form stable complexes. In biomedicine, chelating agents are often used to treat heavy metal poisoning or to remove excess metals from the body. They work by binding to the metal ions and forming a complex that can be easily excreted from the body.
Overall, phosphate binders and chelating agents are important tools in managing conditions related to phosphate imbalance or heavy metal toxicity, respectively.
Drug Target R&D Trends for Sevelamer Hydrochloride
According to Patsnap Synapse, as of 11 Sep 2023, there are a total of 18 Phosphates drugs worldwide, from 52 organizations, covering 17 indications, and conducting 436 clinical trials.
The analysis of target Phosphates reveals a competitive landscape with several companies showing promising growth and R&D progress. Sanofi emerges as a key player with the highest number of drugs in the approved phase. The approved indications for target Phosphates cover a wide range of medical conditions related to phosphate metabolism and kidney function. Small molecule drugs dominate the development phase, indicating their potential market availability. The United States, Japan, and the European Union lead in the development of target Phosphates, with China also making progress. Overall, the current competitive landscape suggests a positive outlook for the future development of target Phosphates in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Sevelamer Hydrochloride is a small molecule drug developed by Genzyme Corp. It is used to target phosphates and is primarily indicated for the treatment of hyperphosphatemia in patients with endocrinology and metabolic diseases. The drug has reached the highest phase of development, with its first approval in the United States in October 1998. Its approval has provided healthcare professionals with an effective therapy to manage hyperphosphatemia and its associated complications.