U.S. Fast-tracks ENHERTU® Assessment for Advanced HER2-Positive Solid Cancer Patients
The US regulatory authorities have given the go-ahead for an expedited review process concerning the additional licensing request submitted by Daiichi Sankyo and AstraZeneca for ENHERTU® (fam-trastuzumab deruxtecan-nxki). This fast-track status was granted with the aim of approving the drug's use for adult individuals battling advanced stages of HER2-positive solid malignancies that are inoperable or have spread beyond the initial site, particularly in cases where patients have undergone previous therapies or lack suitable alternative medical solutions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The American Food and Drug Administration accelerates its examination of drug submissions that could indicate a major leap forward in current therapies. This Priority Review process is triggered when there's a possibility to advance in terms of effectiveness or safety, prevent critical illnesses, or enhance adherence to treatments by patients.
ENHERTU, a targeted therapy against the HER2 protein, is a creation of a partnership between Daiichi Sankyo and AstraZeneca. The duo anticipates a pivotal verdict by the FDA by the deadline of May 30, 2024, based on the Prescription Drug User Fee Act (PDUFA). Following an August 2023 acknowledgment as a Breakthrough Therapy for ENHERTU in treating widespread solid tumors positive for HER2, the FDA has now accelerated its review.
Under review is the sBLA, which is part of the FDA's accelerated programs known as the Real-Time Oncology Review and Project Orbis. These frameworks are intended to fast-track the availability of oncologic treatments with demonstrated safety and efficacy. The innovative RTOR approach lets the FDA begin evaluating parts of a new drug application in advance of the final complete submission, while Project Orbis involves multiple countries' health authorities to streamline the review processes for new cancer drugs.
Dr. Ken Takeshita, who leads Global Research & Development at Daiichi Sankyo, remarked on the extensive clinical gains witnessed in treating HER2 positive solid tumors in the wide-ranging DESTINY-PanTumor02 study. Continuous insights from ENHERTU's extensive clinical trials point to its significant potential to treat a broader spectrum of conditions. With potential approval, ENHERTU is poised to be a first-of-its-kind HER2 targeting and antibody-drug conjugate therapy, designed without restriction to tumor type—poised to offer a fresh avenue of therapy to patients in need.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
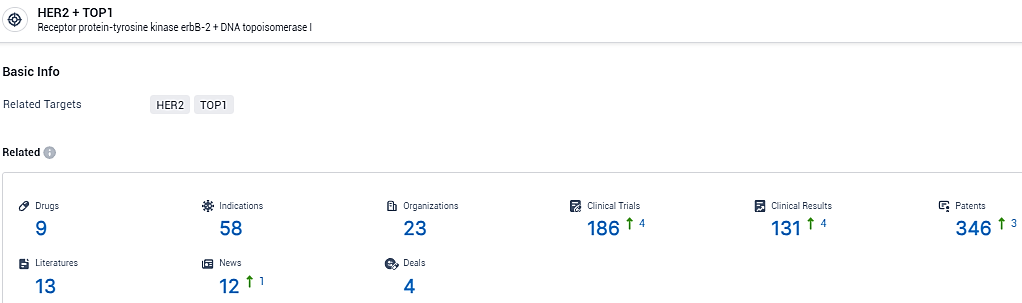
According to the data provided by the Synapse Database, As of January 31, 2024, there are 9 investigational drugs for the HER2 and TOP1 target, including 58 indications,23 R&D institutions involved, with related clinical trials reaching 186, and as many as 346 patents.
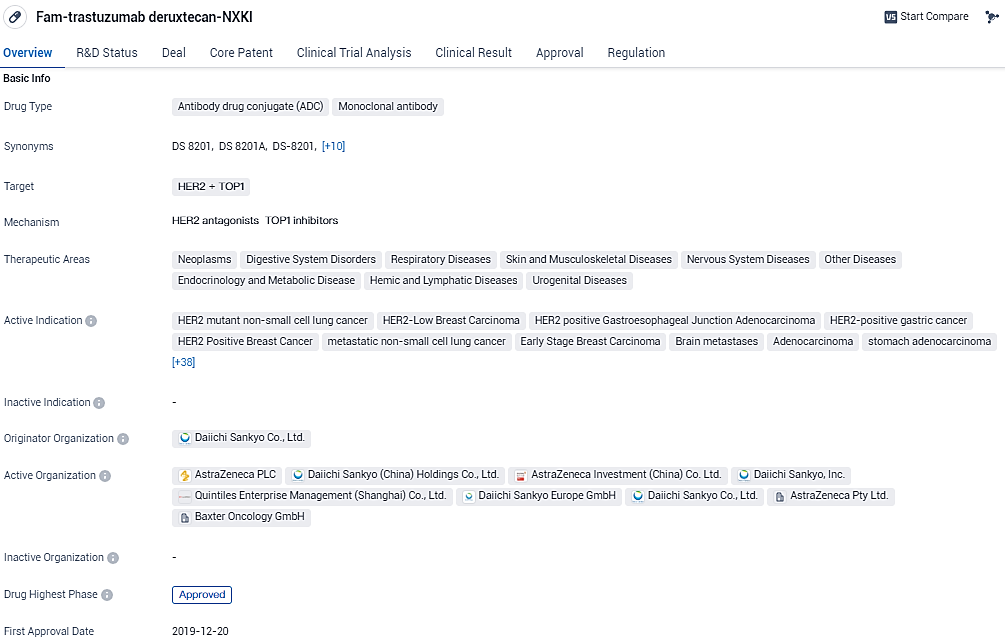
ENHERTU is an ADC and monoclonal antibody that targets HER2 and TOP1. It has been approved for the treatment of various cancers and has received regulatory designations to expedite its development and approval process. The drug's approval in the United States in 2019 marks an important milestone in its availability for patients.