U.S. FDA Fast-tracks Approval for Gilead's Livdelzi in Treating Biliary Cholangitis
Gilead Sciences, Inc. (Nasdaq: GILD) has revealed that the U.S. Food and Drug Administration (FDA) has provided accelerated approval for Livdelzi® (seladelpar) as a treatment for primary biliary cholangitis (PBC). This medication can be used alongside ursodeoxycholic acid (UDCA) in adult patients who do not respond adequately to UDCA or as a standalone treatment for those who cannot tolerate UDCA. Livdelzi is not advised for patients with decompensated cirrhosis or those who may develop it.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The fast-tracked authorization was primarily predicated on data from the crucial placebo-controlled Phase 3 RESPONSE trial. Within this study, 62% of individuals receiving Livdelzi reached the principal endpoint of composite biochemical response at month 12, compared to 20% of those on placebo. Treatment with Livdelzi resulted in the normalization of alkaline phosphatase (ALP) levels—a cholestatic indicator predictive of liver transplant necessity and mortality—in 25% of the participants by month 12. This effect was absent in any participants receiving the placebo. A significant secondary endpoint, the change in pruritus score from baseline at month 6, showed that patients treated with Livdelzi experienced a statistically significant alleviation in pruritus relative to the placebo group.
The FDA sanctioned Livdelzi via accelerated approval based on its efficacy in reducing ALP. Improvements in survival rates or prevention of liver decompensation events have not been demonstrated. The ongoing approval of Livdelzi for the sanctioned indication may depend on validation and delineation of clinical benefit in confirmatory trials.
“There is an increasing number of PBC diagnoses, affecting a diverse population in terms of age, gender, race, and ethnicity. Common symptoms for those with PBC include relentless itching and severe fatigue, which is worsened by nocturnal itching,” stated Carol Roberts, President of The PBCers Organization. “The introduction of a new treatment option that can mitigate this intense itching and improve biomarkers of active liver disease marks a significant advancement for our community.”
PBC is an uncommon, chronic, autoimmune disorder of the bile ducts, affecting about 130,000 Americans, primarily women, and can lead to liver damage and potential liver failure if left untreated. Presently, there is no cure for the disease. Livdelzi, an oral peroxisome proliferator-activated receptor (PPAR) delta agonist, or delpar, aims to challenge the existing PBC treatment standards, which often fall short for many patients who experience suboptimal treatment responses, thus risking further liver damage. Livdelzi has shown sustained efficacy and safety throughout its robust development, including the ability to normalize ALP levels in some PBC patients. Given the significance of ALP levels as a key surrogate marker for disease progression in PBC, healthcare professionals are increasingly viewing ALP normalization as a critical treatment objective.
“Individuals living with PBC have long awaited advancements in treatment. The current approval of Livdelzi, with its unique profile, offers a vital new option,” remarked Daniel O’Day, Chairman and CEO of Gilead Sciences. “We look forward to leveraging Gilead’s extensive experience in liver disease to deliver this promising new treatment to all those in need.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
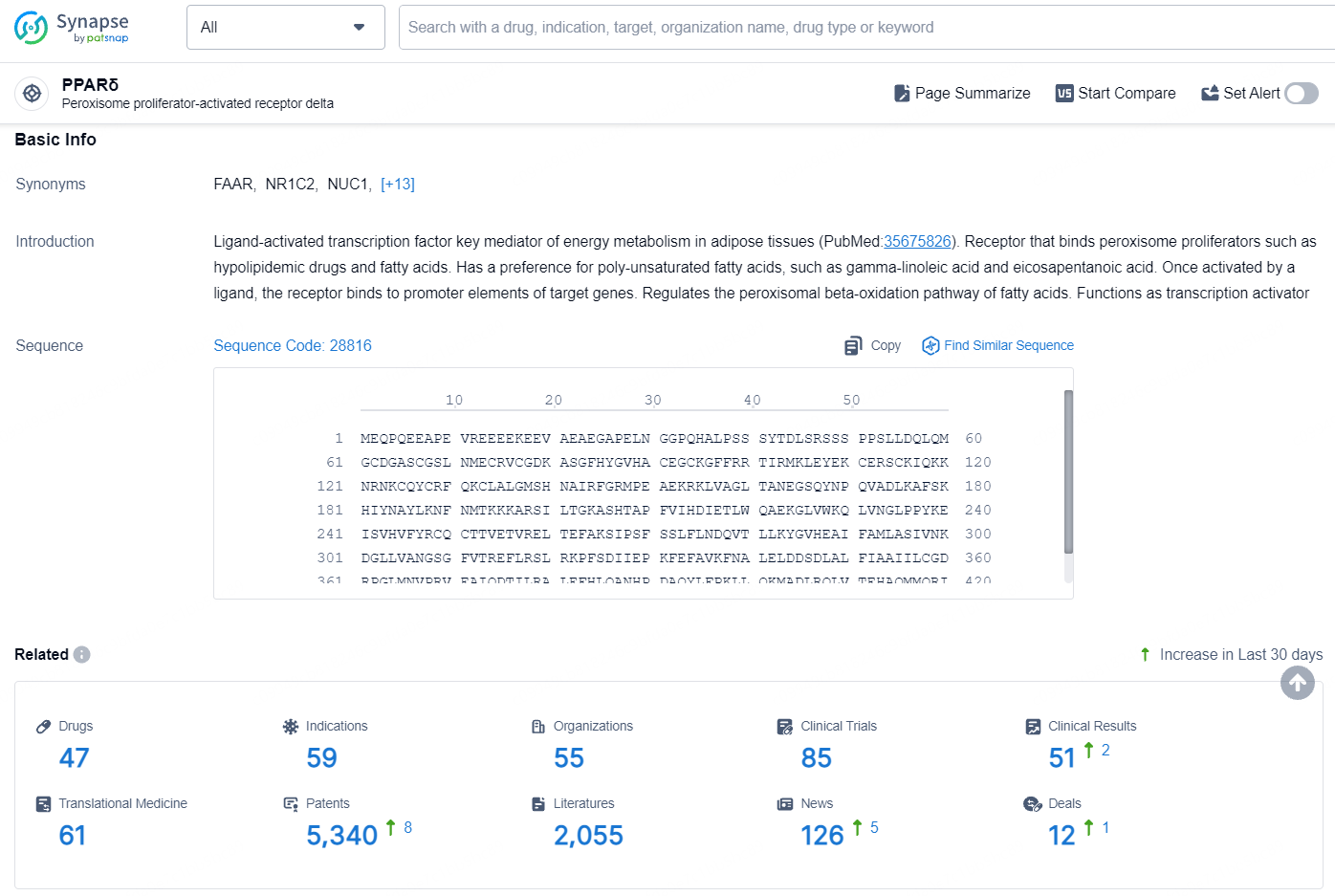
According to the data provided by the Synapse Database, As of August 19, 2024, there are 47 investigational drugs for the PPARδ target, including 59 indications, 55 R&D institutions involved, with related clinical trials reaching 85, and as many as 5340 patents.
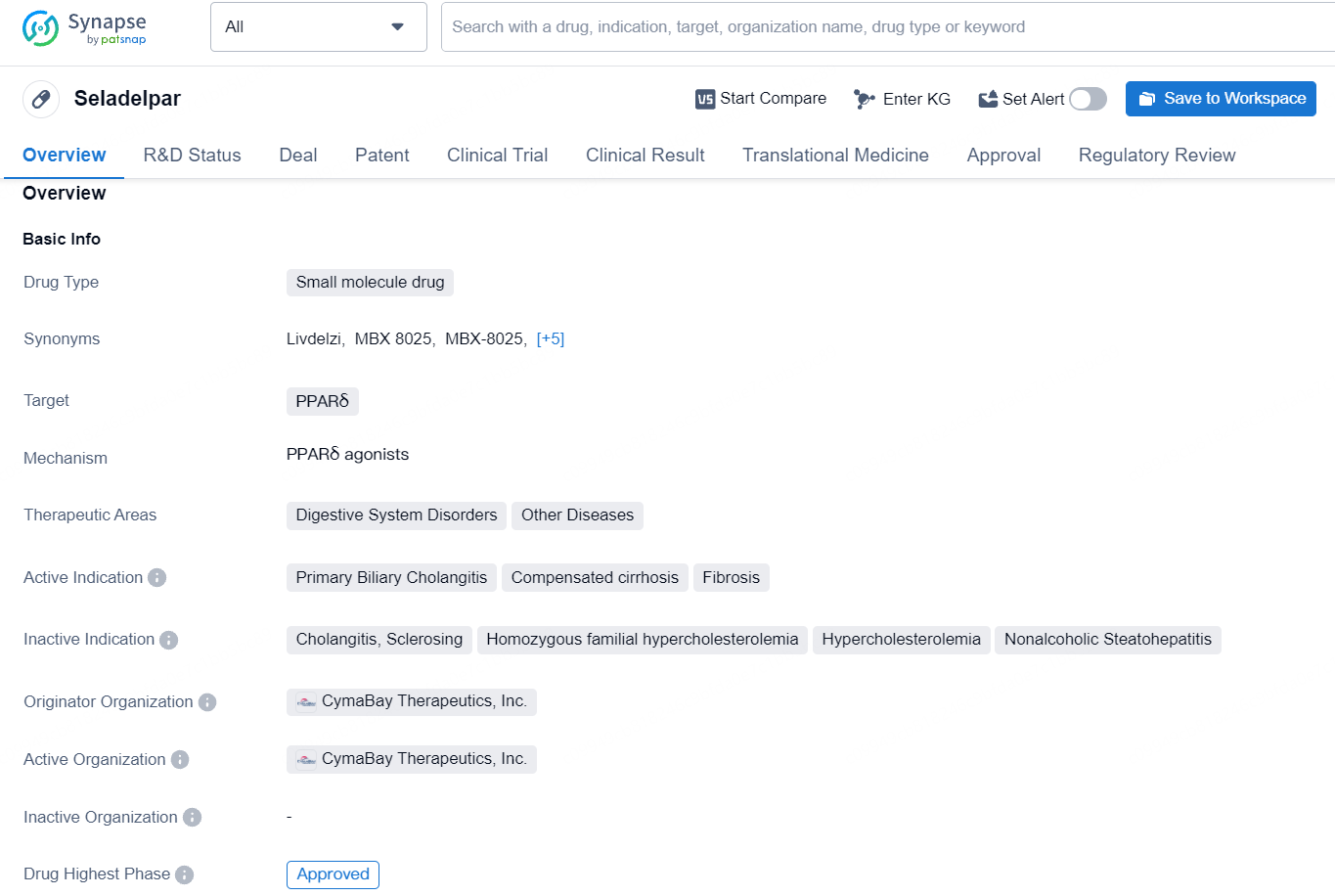
Seladelpar is a small molecule drug that targets PPARδ and is used in the therapeutic areas of Digestive System Disorders and Other Diseases. Its active indications include Primary Biliary Cholangitis, Compensated cirrhosis, and Fibrosis. The drug's originator organization is CymaBay Therapeutics, Inc. and it has achieved its highest phase of approval, with the first approval date occurring in 2024-08 in the United States.