Incyte and Syndax Declare FDA Clearance for Niktimvo™ (axatilimab-csfr) to Treat Chronic GVHD
Incyte (Nasdaq:INCY) and Syndax Pharmaceuticals (Nasdaq:SNDX) announced that Niktimvo™ (axatilimab-csfr), an anti-CSF-1R antibody, has been approved by the U.S. Food and Drug Administration (FDA). This approval applies to the treatment of chronic graft-versus-host disease (GVHD) in adult and pediatric patients weighing a minimum of 40 kg (88.2 lbs.), who have not responded to a minimum of two previous systemic therapies.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The endorsement of Niktimvo signifies a substantial therapeutic breakthrough for individuals with chronic GVHD who have not responded to at least two prior treatments.
“With the endorsement of Niktimvo, patients struggling with chronic GVHD after previous treatment failures now have access to a new treatment option that features a unique mechanism of action aimed at mitigating the severe and harrowing complications of this condition,” stated Hervé Hoppenot, Chief Executive Officer at Incyte. “This is Incyte’s second approved therapy for chronic GVHD, highlighting our ongoing dedication to developing innovative medicines for patients with this disease and supporting the medical community.”
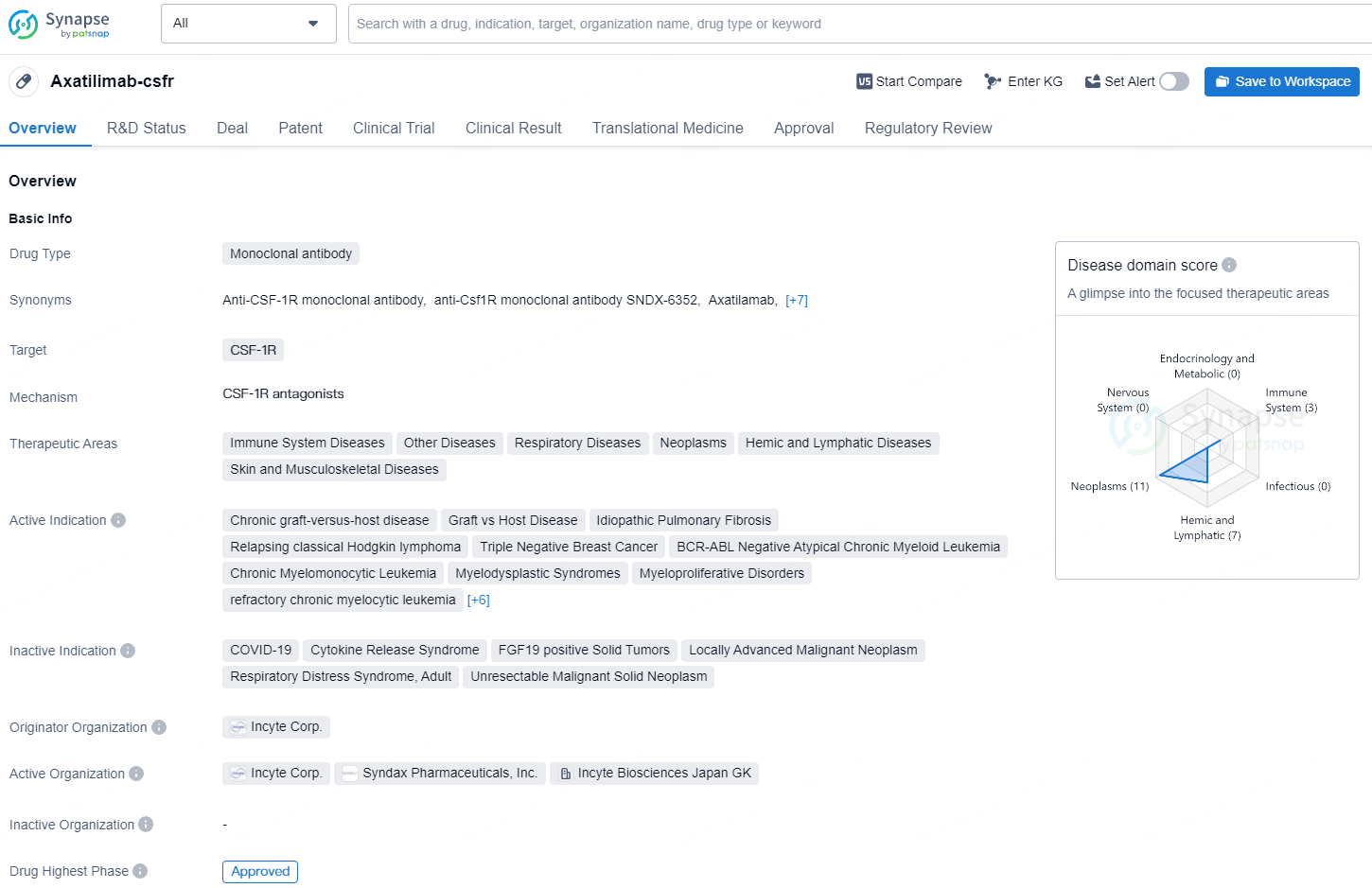
Chronic GVHD is a grave condition that can arise following an allogeneic stem cell transplant (the transfer of stem cells from a donor) where the transplanted cells trigger an immune response against the recipient's organs. Chronic GVHD is a primary cause of significant morbidity and mortality post-allogeneic stem cell transplant, occurring in approximately 42% of transplant recipients and affecting roughly 17,000 individuals in the U.S. Of the patients who develop chronic GVHD, nearly half require at least three lines of therapy, stressing the need for more effective treatment solutions.
“The approval of Niktimvo marks an essential therapeutic advancement for chronic GVHD patients who have failed a minimum of two prior therapies,” remarked Michael A. Metzger, Chief Executive Officer at Syndax. “We are excited to offer this pioneering anti-CSF-1R antibody to patients needing new therapeutic options, and we will continue to investigate axatilimab’s potential in combination with other standard treatments for chronic GVHD and other conditions.”
The FDA approval was granted based on data from the global AGAVE-201 trial, which assessed the safety and effectiveness of Niktimvo in 241 adult and pediatric patients with refractory chronic GVHD who had previously undergone at least two systemic therapies. The trial achieved its primary endpoint across all cohorts receiving Niktimvo. Study results demonstrated lasting responses across all examined organs and patient subgroups. Among the 79 patients who received the approved dose of 0.3 mg/kg every two weeks, 75% achieved an overall response rate (ORR) within six months, with a median time to response of 1.5 months. Additionally, 60% maintained their response for 12 months, measured from initial response to new systemic therapy or death, according to the Kaplan-Meier estimate. The trial also succeeded in a key exploratory endpoint with 56% of patients reporting a ≥7-point improvement in the modified Lee Symptom Scale (mLSS) score. Complete and partial organ-specific responses were observed in all organs affected by chronic GVHD, including the lower GI, upper GI, esophagus, joints/fascia, mouth, lungs, liver, eyes, and skin.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
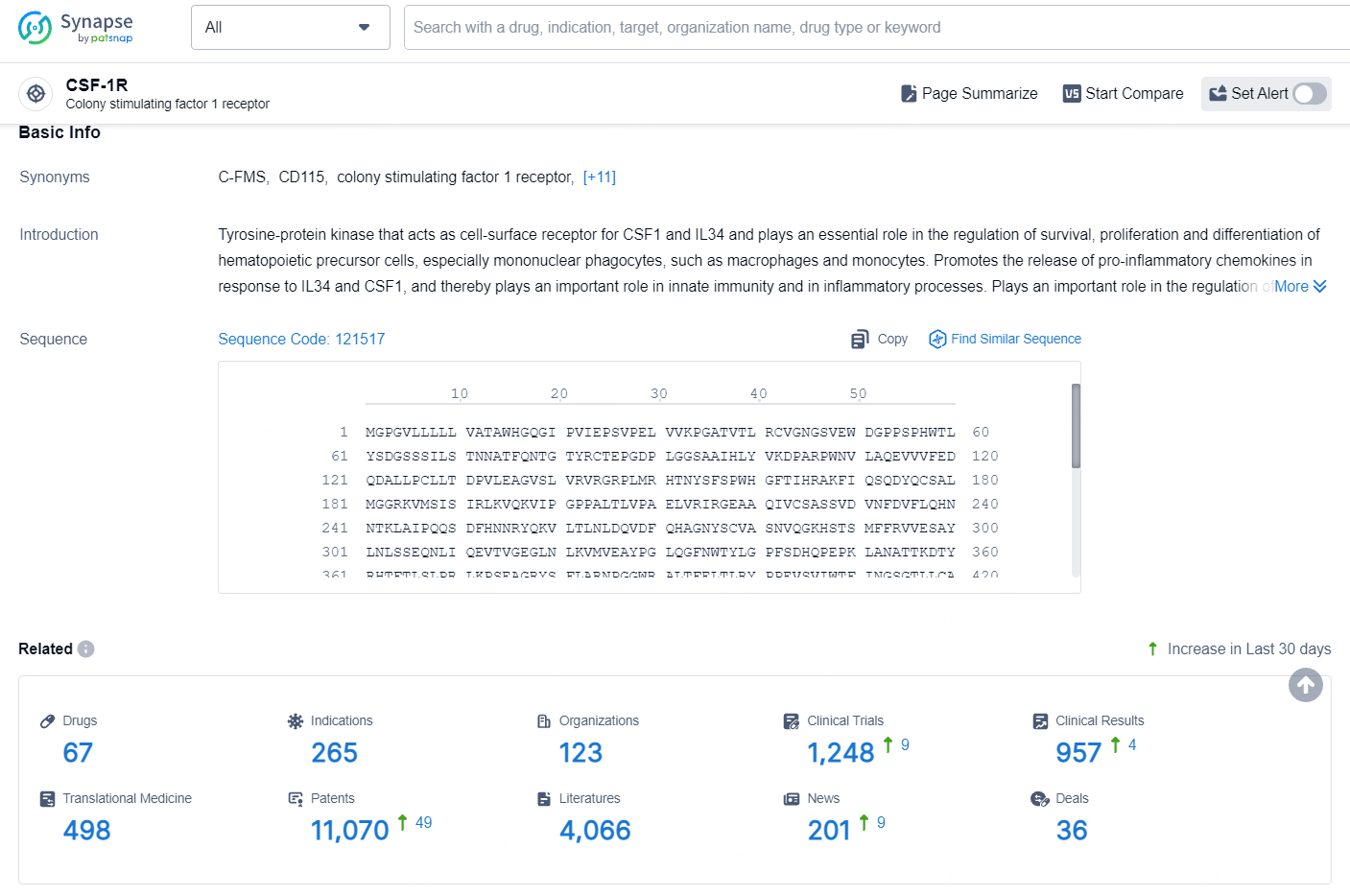
According to the data provided by the Synapse Database, As of August 19, 2024, there are 67 investigational drugs for the CSF-1R target, including 265 indications, 123 R&D institutions involved, with related clinical trials reaching 1248, and as many as 11070 patents.
Axatilimab-csfr is a monoclonal antibody drug that targets CSF-1R and has been approved for various therapeutic areas, including immune system diseases, respiratory diseases, neoplasms, and hemic and lymphatic diseases. It has active indications for a range of conditions such as chronic graft-versus-host disease, idiopathic pulmonary fibrosis, Hodgkin lymphoma, triple-negative breast cancer, and various types of leukemia and solid tumors.