Ventus Therapeutics Announces Phase 1 Results for Brain-Targeting Inhibitor VENT-02
Ventus Therapeutics, a clinical-stage biopharmaceutical firm that employs its unique ReSOLVE™ structural biology and computational chemistry platform, disclosed outcomes from its Phase 1 clinical study regarding VENT-02.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
VENT-02 is a newly developed, orally administered, brain-penetrant NLRP3 inhibitor. This initial phase trial assessed the pharmacodynamics, pharmacokinetics, safety, and tolerability of VENT-02 at various single and multiple ascending doses in a group of healthy adult participants.
"We are gratified by the outcomes of the trial where VENT-02 showed extensive therapeutic range and full target coverage, highlighting its capability to address both central nervous system ailments and peripheral diseases that currently lack effective treatments," stated Xavier Valencia, M.D., Chief of Clinical Development at Ventus.
Xavier Valencia further noted, “The upcoming Phase 1b study of VENT-02 involving Parkinson’s disease patients intends to explore various doses and administration schedules compared to a placebo, as well as examine various markers linking inflammation to disease processes. Given the encouraging pharmacodynamic data and safety profiles observed in the initial Phase 1 study, and our ongoing clinical strategy, we are optimistic about VENT-02’s potential to achieve leading efficacy in its class."
During this initial Phase 1 study, VENT-02 was found to be generally safe, exhibiting no dose-limiting toxic effects or serious adverse incidents, with only minor or moderate side effects like headaches and nausea at doses significantly higher than therapeutic levels.
VENT-02 achieved full engagement with its target, inhibiting 100% of IL-1ß in blood in an ex vivo assay, demonstrating notable drug concentration in the CSF persisting for 24 hours, and effectively reducing inflammatory markers such as hsCRP. Considering the drug’s half-life and target engagement demonstrated during the trial, VENT-02 shows promise for daily dosing.
“The outstanding attributes of VENT-02 reflect Ventus’ forefront work on NLRP3, including proven concept in preclinical evaluations across models relevant to disease, biomarker validation, and partnerships with academic entities as well as the Michael J. Fox Foundation,” mentioned Marcelo Bigal, M.D., Ph.D., President and CEO of Ventus.
Marcelo Bigal elaborated, “Given these achievements, we are moving forward with introducing VENT-02 to Parkinson’s disease and other specific diseases where the CNS is significantly involved. Our creatively designed upcoming trials aim to yield informative results that will decrease developmental risks. Moreover, these trials are expected to allow us to assess the broader implications of NLRP3 inhibition across various patient groups afflicted with severe conditions.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
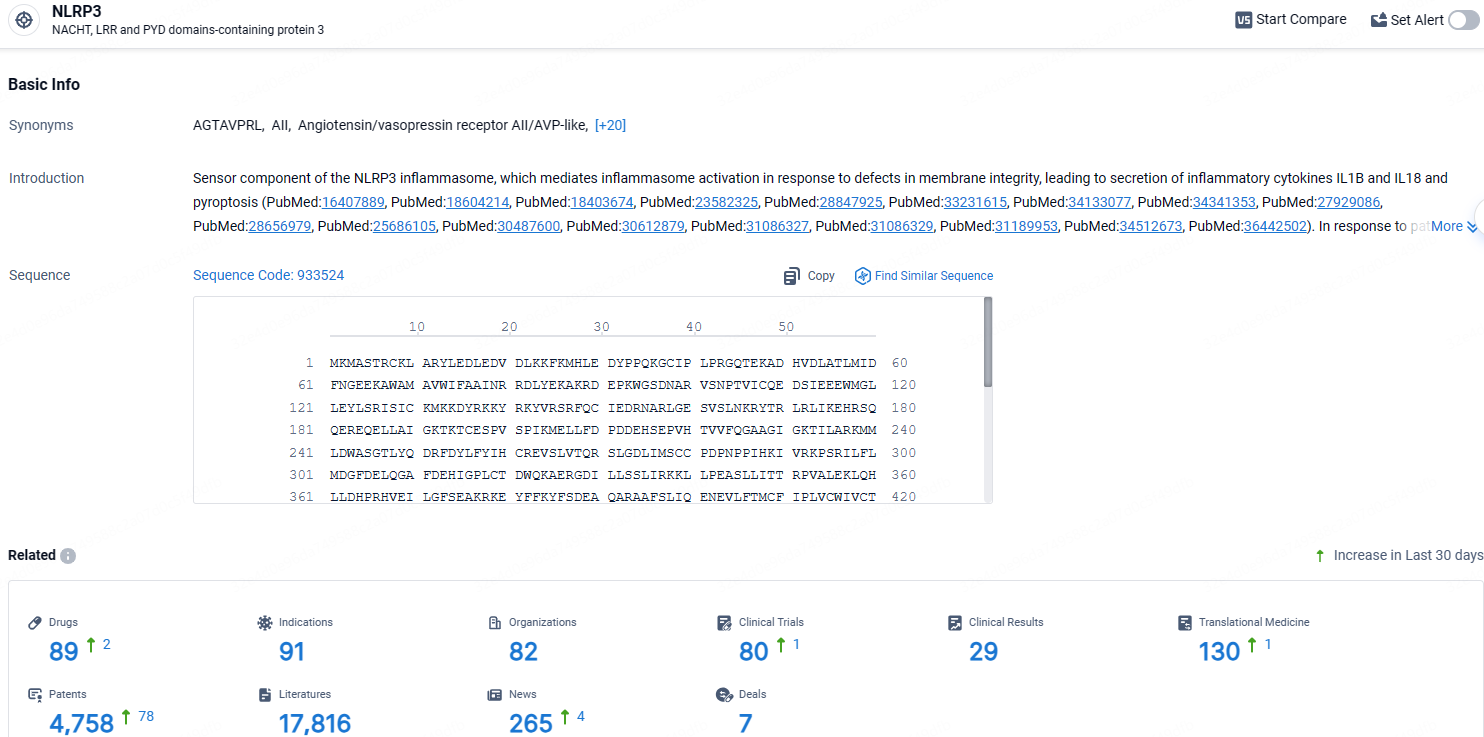
According to the data provided by the Synapse Database, As of April 18, 2024, there are 89 investigational drugs for the NLRP3 target, including 91 indications, 82 R&D institutions involved, with related clinical trials reaching 80, and as many as 130 patents.
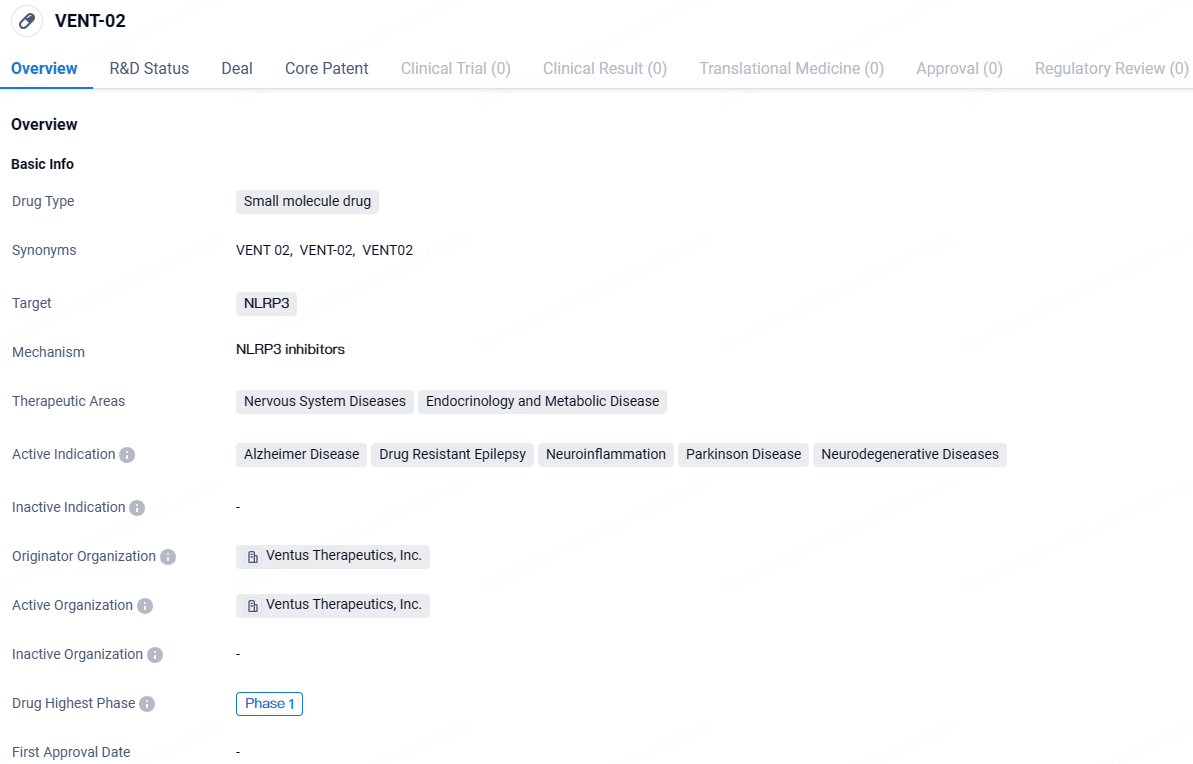
VENT-02 targets NLRP3 and is being developed for the treatment of nervous system diseases and endocrinology and metabolic diseases. The drug is currently in Phase 1 of clinical development and shows potential for addressing conditions such as Alzheimer's disease, drug-resistant epilepsy, neuroinflammation, Parkinson's disease, and other neurodegenerative diseases.