48-Week Frexalimab Trial Shows Sustained Multiple Sclerosis Treatment Promise
Sanofi's antibody targeting CD40L, known as frexalimab, has shown consistent disease activity decrease and was well tolerated over an extended period nearing one year in subjects with relapsing multiple sclerosis. This information is set to be shared at the American Academy of Neurology's 2024 Annual Meeting, which will take place in Denver, Colorado, USA. Earlier, findings from a 12-week, double-blind phase of the study had been documented in The New England Journal of Medicine.
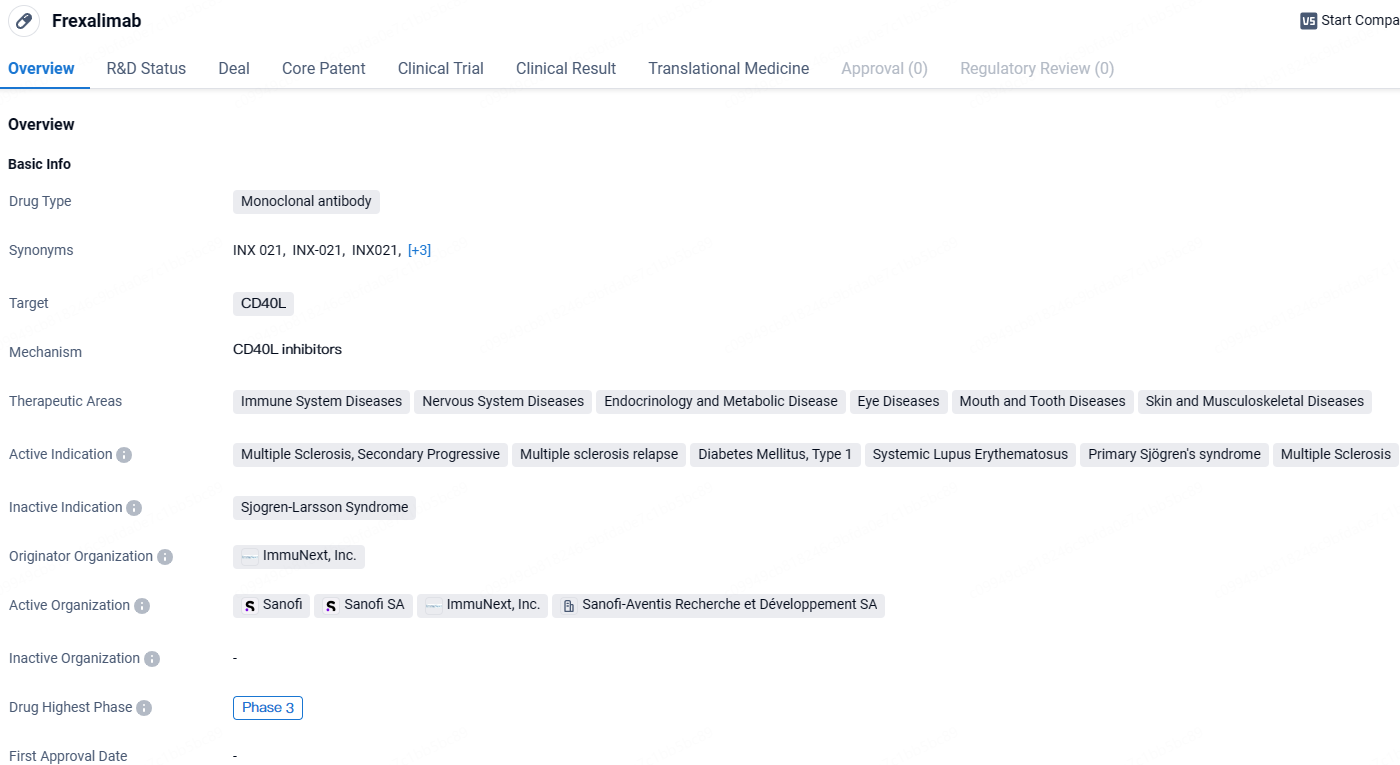
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The data collected at 48 weeks indicate that frexalimab treatment led to a continuous decrease in lesion count along with persistent suppression of disease activity. According to the early clinical findings, the rate of annual relapses was remarkably low, highlighting the potential of targeting CD40L in MS treatment. "These findings support ongoing efforts to advance frexalimab's development as a promising, highly effective treatment for relapsing MS," stated Patrick Vermersch, MD, PhD, from University of Lille, CHU Lille, France.
From the start of the study, following an initial 12-week double-blind stage, 97% of the participants progressed to the open-label extension in a phase 2 study. By the week 48 conclusion, 87% of those treated with frexalimab, across both high- and low-dose groups and including those who moved from the placebo group, remained in the study.
In the open-label extension (OLE), individuals receiving high doses (n=50) and low doses (n=49) of frexalimab were administered 1200 mg intravenously every four weeks and 300 mg subcutaneously every two weeks, respectively. Participants initially on placebo were transferred to the respective high or low dose frexalimab treatment arms, involving 12 and 14 participants respectively.
“Frexalimab introduces a groundbreaking, potential first-in-class treatment for multiple sclerosis, specifically addressing areas of significant unmet needs," Erik Wallström, MD, PhD, Global Head of Neurology Development at Sanofi, noted. "Our commitment leverages extensive knowledge in neurology to tackle both neuroinflammation and neurodegeneration, enhancing the quality of life for those affected by multiple sclerosis.”
Frexalimab (SAR441344) is an investigational second-generation anti-CD40L antibody and a potentially first-in-class treatment. It inhibits the CD40/CD40L costimulatory pathway, crucial for both adaptive and innate immune functions. Its unique action potentially allows it to deal with both immediate and long-term neuroinflammation in MS without reducing lymphocyte numbers.
Sanofi has secured exclusive rights to develop frexalimab through a licensing agreement with ImmuNext Inc. Current evaluations include phase 3 clinical trials for Multiple Sclerosis and phase 2 trials for immunology issues and Type 1 Diabetes. However, its safety and efficacy are yet to be assessed by any regulatory agencies.
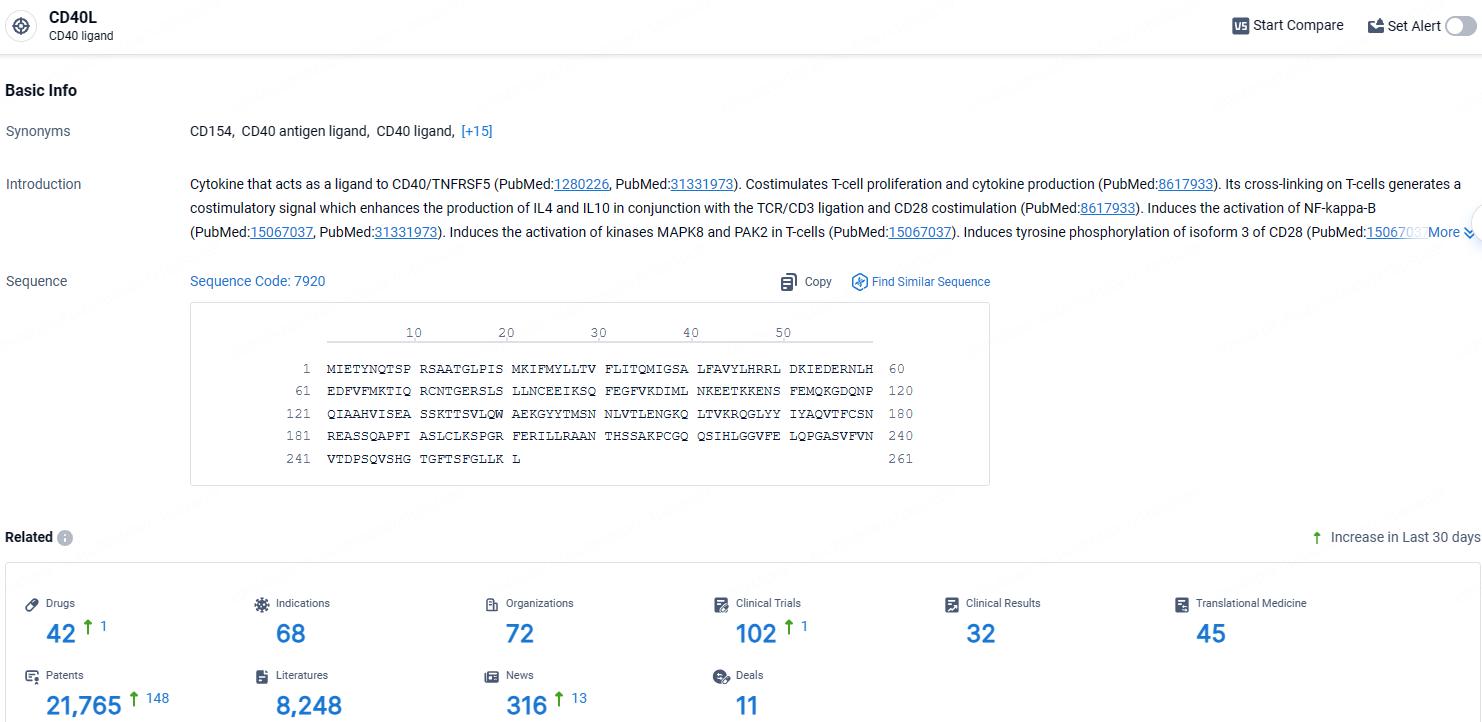
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of April 18, 2024, there are 42 investigational drugs for the CD40L target, including 68 indications, 72 R&D institutions involved, with related clinical trials reaching 102, and as many as 45 patents.
Frexalimab appears to be a monoclonal antibody drug with a broad therapeutic potential in various disease areas. Its specific targeting of CD40L and its advancement to Phase 3 globally indicate that it may hold promise as a treatment option for multiple sclerosis and other related conditions. Frexalimab has received IND approval, which means that it has been granted permission to conduct clinical trials in the country. This suggests that the drug has gained recognition and interest from regulatory authorities in China.