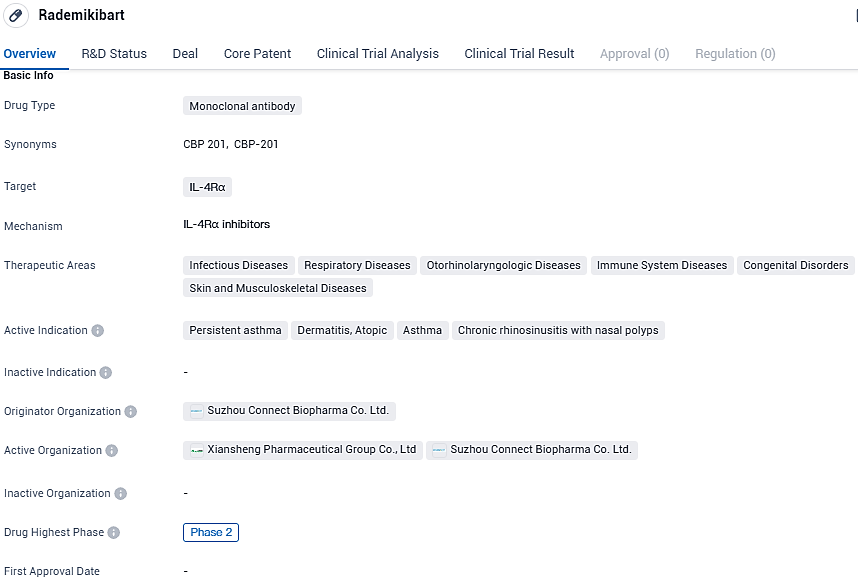
Connect Biopharma reports positive Phase 2b results for Rademikibart in moderate to severe adult asthma
Connect Biopharma Holdings Limited, an international biopharmaceutical firm engaged in the clinical advancement of treatments aimed at enhancing the well-being of individuals suffering from persistent inflammatory conditions, has declared the successful initial outcomes from an international Phase 2b study. This study assessed the effectiveness and safety profile of rademikibart in treating adults experiencing moderate to severe persistent asthma.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The international Phase 2b research encompassed a broad-based, multi-institutional, randomized, double-masked, placebo-managed scheme executed across 79 locations spanning the United States, Poland, Hungary, China, and South Korea. A total of 322 participants were distributed in equal fractions of 1:1:1 to receive either rademikibart at a dose of 150 mg biweekly following an initial 600 mg dosage, rademikibart at 300 mg biweekly post an initiating dose of 600 mg, or a placebo. The majority, approximating two-thirds, of the participants who underwent randomization received treatment within the U.S.
While the research wasn't primarily designed to identify variances in flare-ups, the administration of rademikibart exhibited positive indications of diminishing such incidents. Over the course of the 24-week investigation, the placebo arm recorded more than half of the total exacerbations. Further, both dosages of rademikibart seemed to contribute to a considerable delay in the occurrence of the initial exacerbation when compared to the placebo.
Dr. Zheng Wei, who is a co-founder and the Chief Executive Officer at Connect Biopharma, expressed excitement regarding the Phase 2b trial outcome. “The international findings manifest a statistically significant and consistent enhancement in pulmonary capability," Zheng Stated. "The data showcases that rademikibart, administered at both the higher and lower doses, was effective starting from the very first week of treatment."
Zheng added, "The favorable outcomes across the globe synergize with the optimistic findings from the crucial trial for atopic dermatitis held in China. These combined results enhance the prospect of rademikibart emerging as an industry-leading new-generation anti-IL-4Rα antibody for the treatment of Th2-axis-driven illnesses."
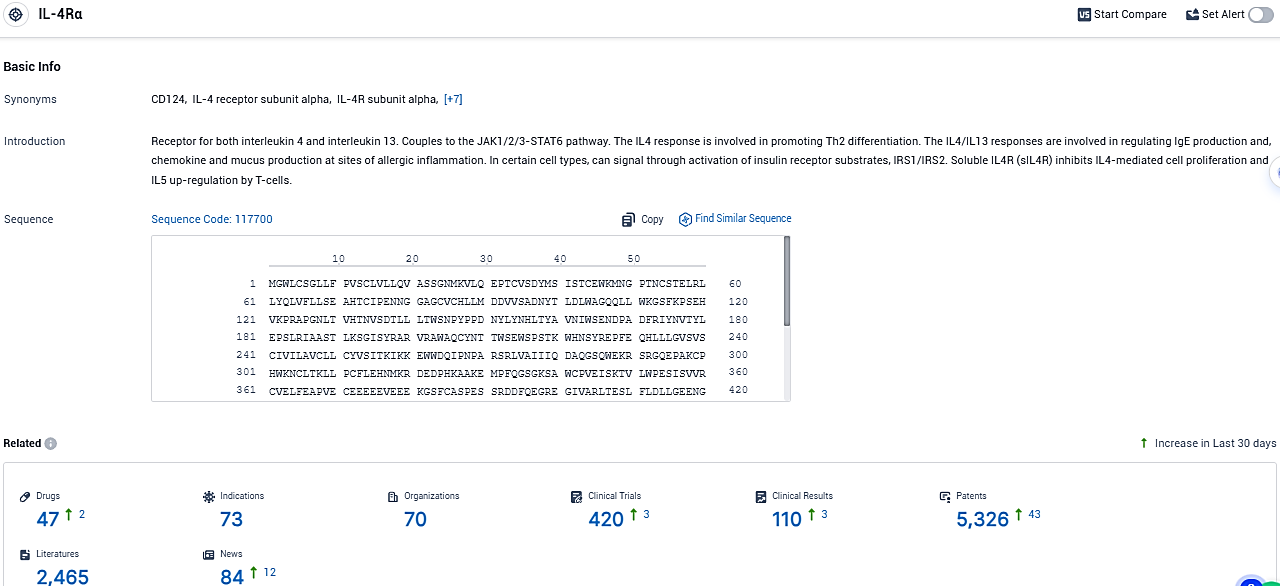
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 19, 2023, there are 47 investigational drugs for the IL-4Rα target, including 73 indications, 70 R&D institutions involved, with related clinical trials reaching 420, and as many as 5326 patents.
Rademikibart shows potential as a therapeutic option for various diseases within the specified therapeutic areas. However, further research and clinical trials are needed to establish its safety and efficacy. The drug's progress to Phase 2 trials suggests that it has shown promising results thus far, but it is important to await further data and analysis to determine its ultimate potential in the pharmaceutical market.