Viking Therapeutics has launched the Phase 2 VENTURE study of the combined GLP-1/GIP receptor agonist VK2735 for obese patients
Viking Therapeutics, Inc., a biomedical enterprise in the clinical phase, concentrating on inventing novel treatments for metabolic and endocrine abnormalities, has recently commenced a Phase 2 clinical evaluation of VK2735. This unique compound, entirely owned by the company, acts as a dual stimulator of the GLP-1 and GIP receptors. The developmental trials for VK2735 are mainly aimed at potentially providing a cure for diverse metabolic issues, including obesity.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The VENTURE Phase 2 trial is designed as a randomized, double-blind, placebo-based study that predicts the levels of safety, tolerability, pharmacokinetics, and the effectiveness of weight-loss across four diverse VK2735 dosages, delivered once per week through subcutaneously. The study spanning 13 weeks will involve about 125 grown-ups suffering from obesity or being overweight with at least one weight-related health condition.
The foremost objective of this research is to ascertain the percentage deviation in body weight from the starting point to the 13th week, with peripheral and exploratory objectives examining a variety of safety and efficacy metrics.
"We eagerly anticipate the commencement of our VENTURE study, following the compelling Phase 1 data of VK2735 released earlier in the year," stated Brian Lian, Viking's CEO and Ph.D. holder. "Defeating obesity remains a healthcare priority since efficient weight-loss treatments can diminish a patient's risk for cardiovascular issues. This 13-week study aims to investigate the safety and ability of VK2735 to promote weight loss."
Most of the adverse events (98%) noted in the Phase 1 trial were classified as light or moderate. Similarly, gastrointestinal-specific adverse happenings (99%) were majorly classified as being light or moderate. Notably, despite the significant activation of the incretin receptor pathways, zero instances of hypoglycemia emerged. The company maintains its belief that the results from Phase 1 suggest that higher dosages can be reached with longer titration intervals.
Viking is further implementing a Phase 1 trial for an innovative, orally administered version of VK2735 as they posit that it could greatly spread the compound's market footprint by providing an option between subcutaneous and oral administrations. The enterprise is expected to release preliminary data from this Phase 1 study during the last quarter of 2023.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
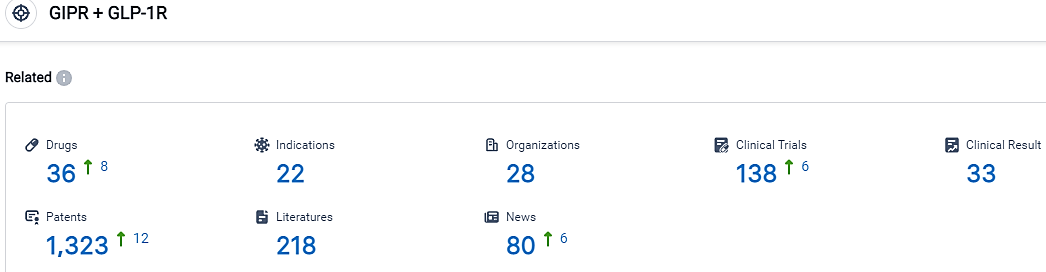 According to the data provided by the Synapse Database, As of September 8, 2023, there are 36 investigational drugs for the GIPR and GLP-1R target, including 22 applicable indications,28 R&D institutions involved, with related clinical trials reaching 138,and as many as 1323 patents.
According to the data provided by the Synapse Database, As of September 8, 2023, there are 36 investigational drugs for the GIPR and GLP-1R target, including 22 applicable indications,28 R&D institutions involved, with related clinical trials reaching 138,and as many as 1323 patents.
The stimulation of the glucagon-like peptide 1 (GLP-1) receptor has been demonstrated to reduce glucose levels, curb hunger, decrease body mass, and enhance insulin sensitivity in individuals suffering from type 2 diabetes, obesity, or both conditions. The U.S. Food and Drug Administration has granted approval to Semaglutide, a GLP-1 receptor activator, which is now available in a range of dosages and forms under the names Ozempic?, Rybelsus?, and Wegovy?. The future for GLP-1 appears bright, with potential progress expected in the therapy of diabetes, obesity, and other metabolic ailments.