What Does Inebilizumab-cdon Do?
Inebilizumab-cdon, commercially known as Uplinza, is an innovative immunomodulatory agent developed by Viela Bio, now a part of Horizon Therapeutics USA. This humanized monoclonal antibody targets CD19-expressing B cells, which are involved in the pathology of neuromyelitis optica spectrum disorder (NMOSD). Inebilizumab-cdon is designated as an orphan drug by the FDA for the treatment of NMOSD, a rare autoimmune disease that affects the optic nerves and spinal cord.The drug is administered intravenously and has been shown to reduce the frequency of relapses in patients with NMOSD who test positive for aquaporin-4 (AQP4) autoantibodies.
Mechanism of Action of Inebilizumab-cdon
Inebilizumab-cdon's mechanism of action involves binding to CD19, a cell surface antigen present on pre-B and mature B lymphocytes. This binding leads to the depletion of CD19-expressing B cells, which are thought to contribute to the inflammation and damage observed in NMOSD. By reducing the number of these B cells, Inebilizumab-cdon is believed to mitigate the disease's progression and decrease the risk of relapse.
How to Use Inebilizumab-cdon
Inebilizumab-cdon is administered via intravenous infusion by a healthcare professional. The initial treatment consists of two 300-mg doses given two weeks apart, followed by subsequent doses every six months. Before each infusion, patients should be evaluated for active infection, and the administration should be delayed until the infection resolves. Premedication with a corticosteroid, an antihistamine, and an antipyretic is recommended to reduce the frequency and severity of infusion reactions.
Side Effects of Inebilizumab-cdon
Inebilizumab-cdon can cause a range of side effects, including infusion reactions, which may present with symptoms such as headache, nausea, somnolence, dyspnea, fever, myalgia, rash, and other signs or symptoms. More serious side effects include the risk of infections, particularly in patients with a history of hepatitis B or tuberculosis. Progressive multifocal leukoencephalopathy (PML), a rare but serious brain infection, has been observed with similar B-cell-depleting therapies and is a concern with Inebilizumab-cdon as well.
Drug Interactions with Inebilizumab-cdon
Concurrent use of Inebilizumab-cdon with other immunosuppressant drugs may increase the risk of infection. It is crucial to inform healthcare providers of all current medications, including prescription and over-the-counter drugs, vitamins, and herbal supplements, as they may interact with Inebilizumab-cdon. Additionally, live or live-attenuated vaccines should not be given during treatment and until B-cell repletion has occurred, as Inebilizumab-cdon may interfere with the safety and effectiveness of vaccines.
In conclusion, Inebilizumab-cdon is a targeted therapy providing a new treatment option for patients with NMOSD. Its ability to deplete specific B cells has shown promise in reducing relapses in this debilitating disease. However, due to its immunomodulatory effects, patients should be closely monitored for potential side effects and drug interactions. The management of NMOSD with Inebilizumab-cdon requires a multidisciplinary approach, with careful consideration of each patient's unique medical history and treatment needs.
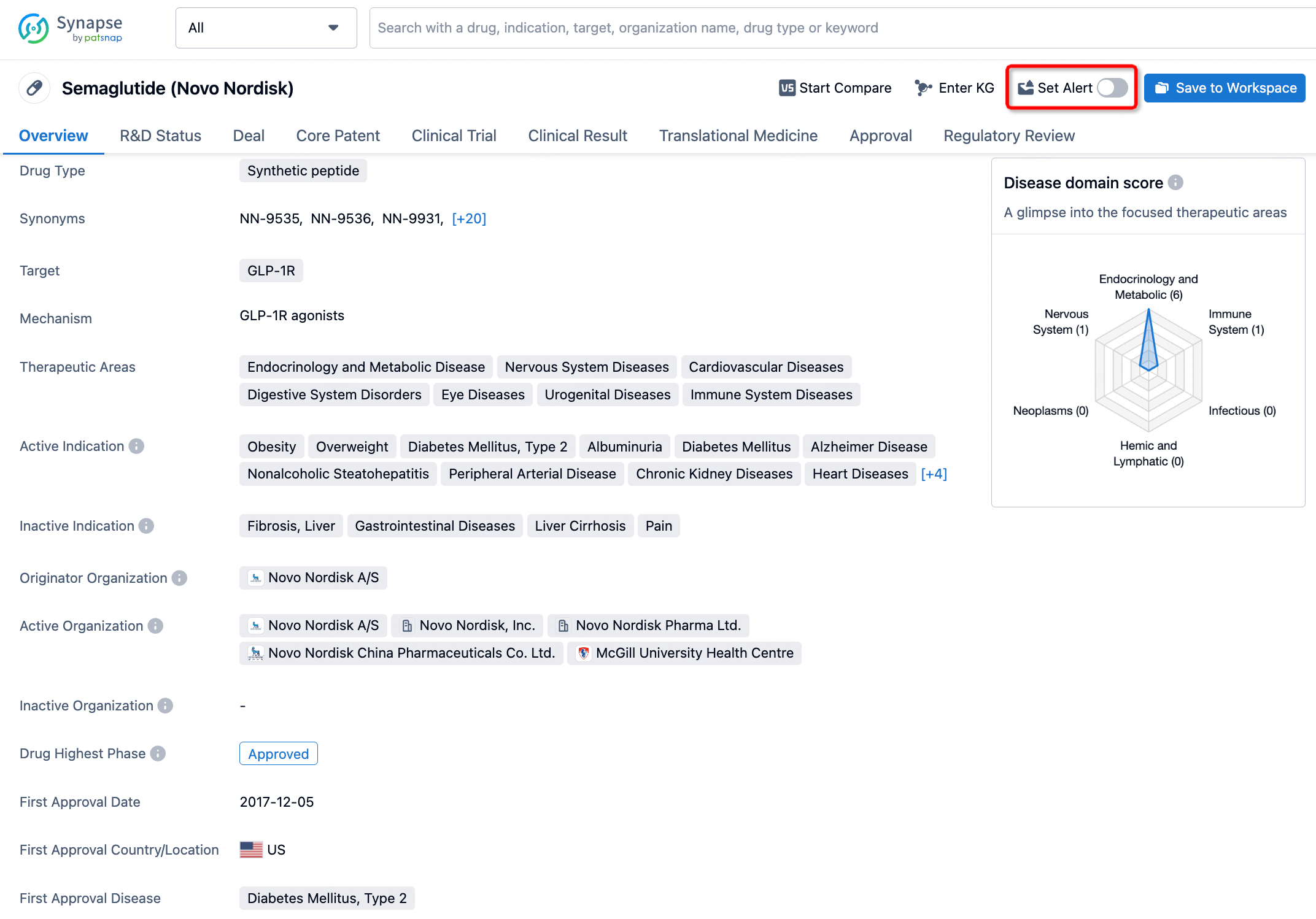
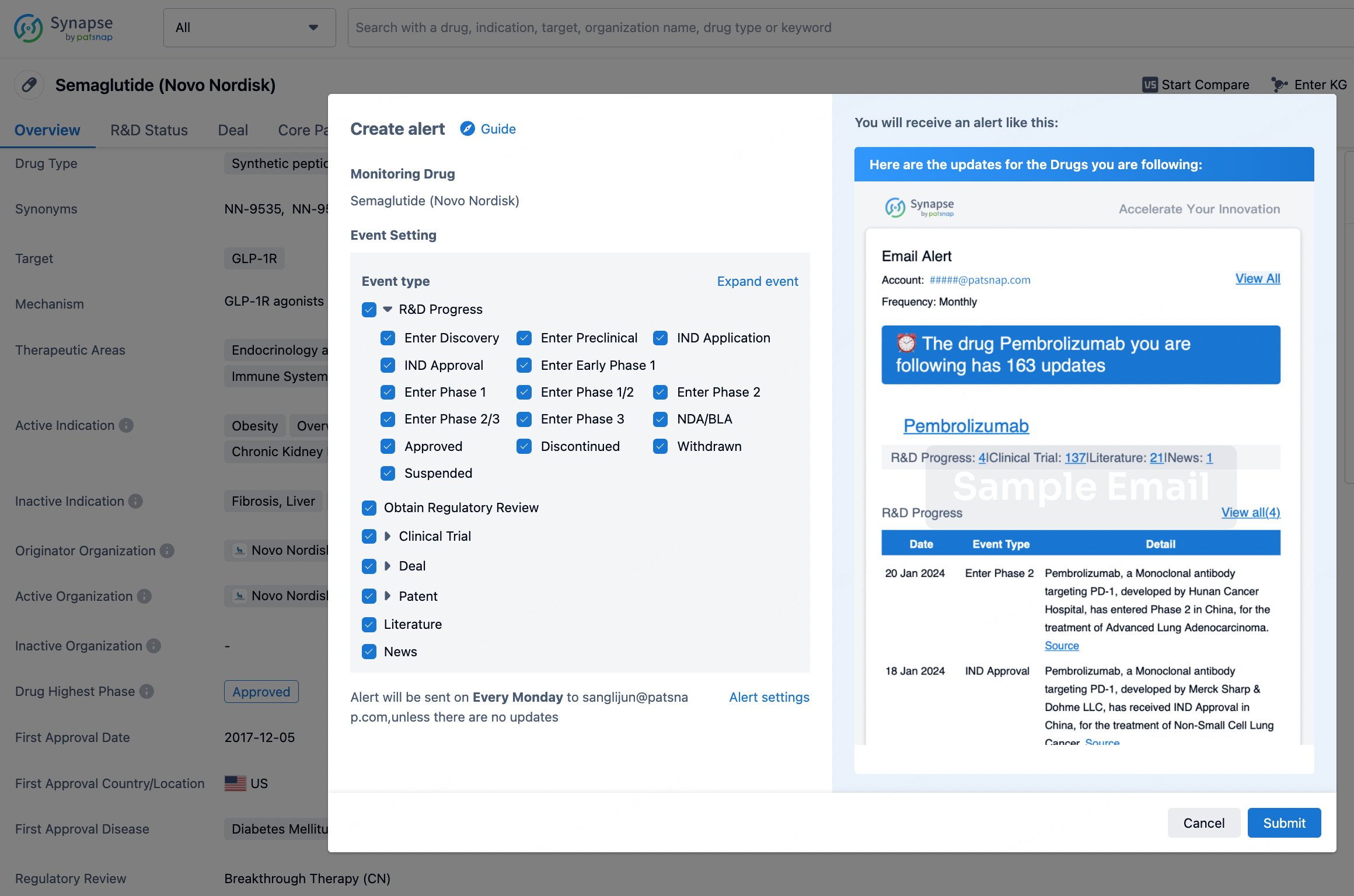
How to obtain the latest development progress of all drugs?
In the Synapse database, you can keep abreast of the latest research and development advances of all drugs anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!