Atsena Therapeutics Announces Positive Early Results in Phase 1/2 XLRS Gene Therapy Study
Atsena Therapeutics, a company at the clinical stage specializing in gene therapy, released encouraging early results from the initial group of the LIGHTHOUSE study. This Phase I/II clinical trial investigates the effects of subretinal injection of ATSN-201 in treating X-linked retinoschisis (XLRS). Atsena is dedicated to harnessing the transformative capacity of genetic medicine to either reverse or prevent vision loss.
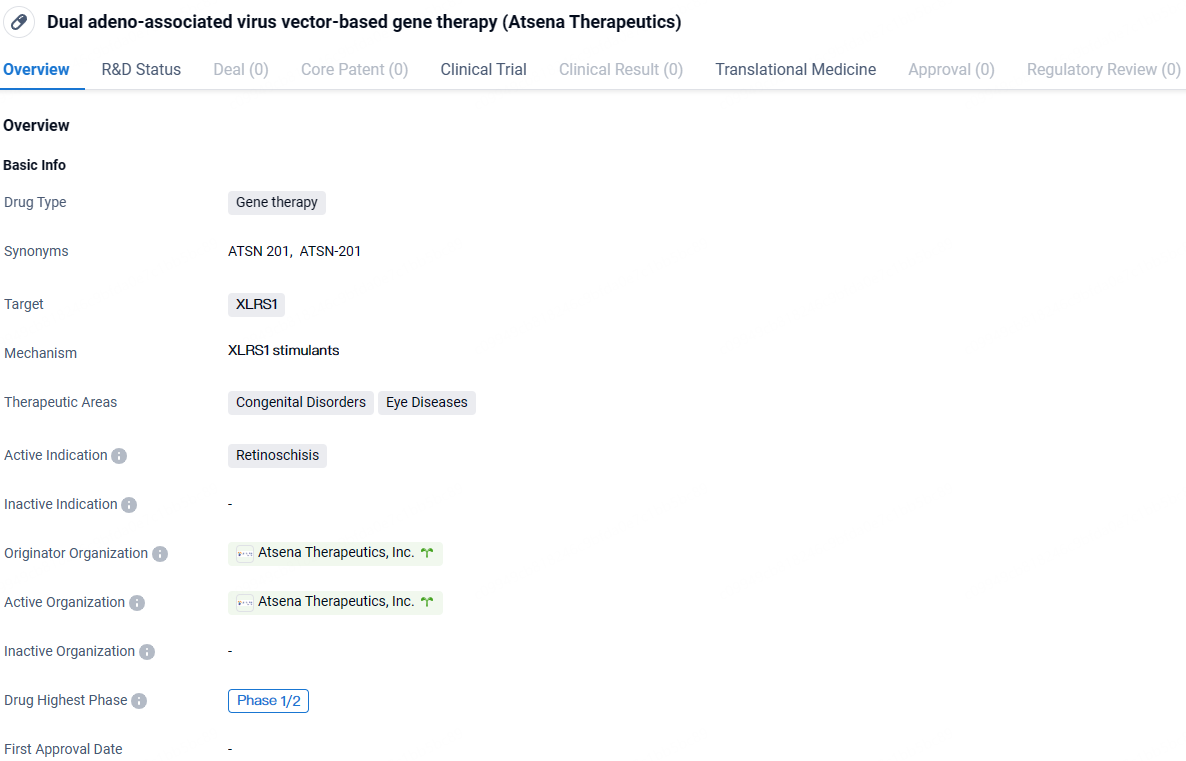
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ATSN-201 employs AAV.SPR, a unique capsid developed by the company, to deliver therapeutic gene expression to photoreceptors in the central retina, bypassing the surgical complications associated with foveal detachment. The LIGHTHOUSE trial is testing ATSN-201 in males aged 6 years and older diagnosed with XLRS due to RS1 gene mutations. This disorder involves the splitting of retinal layers, known as schisis, leading to decreased visual acuity that glasses cannot correct and resulting in progressive vision loss, ultimately causing blindness. Approximately 30,000 males in the United States and Europe are affected by XLRS, for which no approved treatments currently exist.
In the initial group of the LIGHTHOUSE trial, two out of three participants exhibited significant schisis resolution starting eight weeks post-treatment. Continued improvement was noted up to week 24, which is the most recent follow-up period reported. Importantly, resolution of the schisis cavities was observed both within and beyond the areas of the subretinal injections, suggesting that AAV.SPR is effectively dispersing from the initial sites of injection, aligning with the anticipated properties of this novel capsid designed for lateral spread.
Preliminary signs of efficacy were also detected through microperimetry assessments, where enhancements in function mirrored the structural resolutions observed. In one case, a patient experienced up to a 14 dB improvement, with 38 spots showing enhancements of more than 7 dB. The FDA regards an enhancement of 7 dB or more across at least five predetermined spots as clinically significant.
Furthermore, ATSN-201 was well received by all participants in the first group of the XLRS study, with no serious adverse effects reported. These findings suggest for the first time that subretinal injections can be administered safely in patients with extensive retinal schisis.
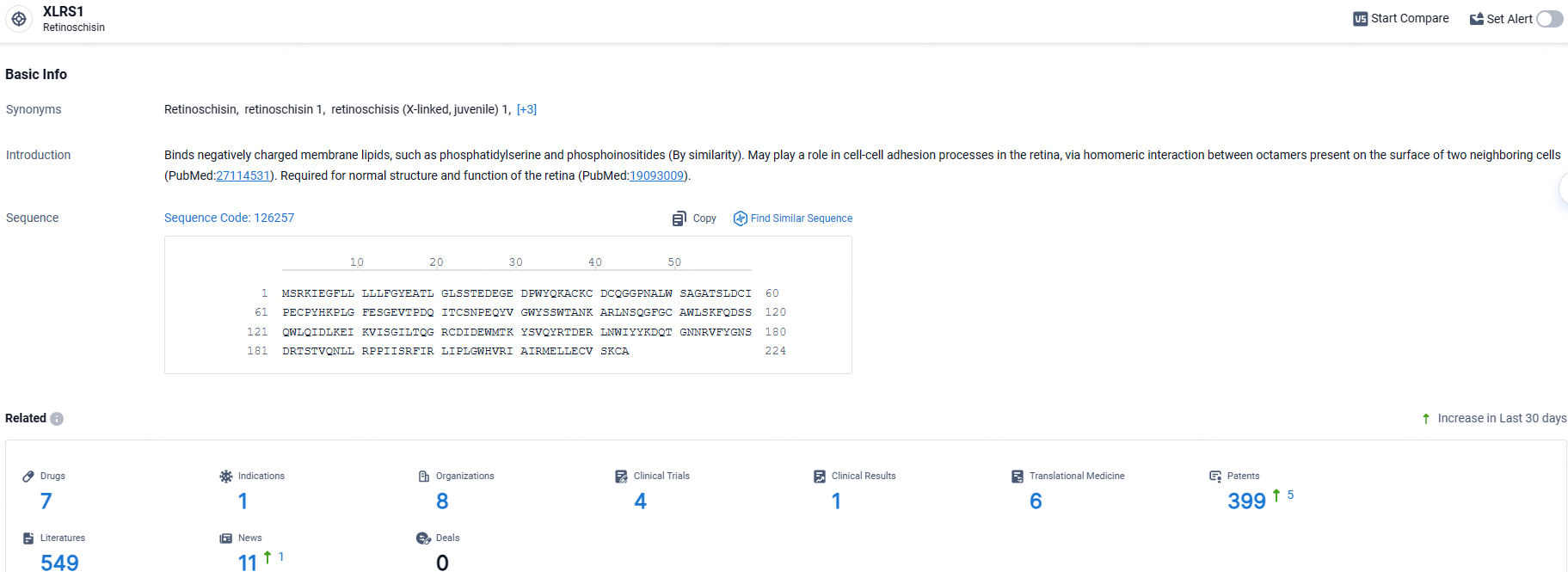
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 9, 2024, there are 7 investigational drugs for the XLRS1 targets, including 1 indications, 8 R&D institutions involved, with related clinical trials reaching 4, and as many as 399 patents.
ATSN-201 is a gene therapy targeting the XLRS1 gene for the treatment of Retinoschisis. With its current highest phase being Phase 1/2, this drug holds promise in the field of biomedicine and may offer a potential treatment option for individuals with this congenital eye disorder.