What ADC-related progress will CStone Pharmaceuticals reveal at the ASCO conference?
CStone Pharmaceuticals is focused on the research and development, as well as the commercialization, of innovative oncological immunotherapies and precision medicine drugs. It has established a rich pipeline consisting of 14 oncology candidate drugs, including four ADC (Antibody-Drug Conjugate) pipelines.
The disclosed CS5001 is an ADC targeting ROR1, featuring a unique design. It only releases the PBD prodrug upon being endocytosed by the tumor cells and reaching the lysosome, where the linker is cleaved by enzymes highly expressed in tumor cells. The activated PBD prodrug then kills the tumor cells. This "dual-control" mechanism of the linker plus prodrug effectively reduces the toxicity associated with traditional PBD payloads, achieving a greater safety margin.

In October 2020, CStone Pharmaceuticals entered into a licensing agreement with LegoChem Biosciences (LCB) for the development and commercialization of CS5001. CS5001 was initially synthesized jointly by LCB and ABL Bio, leading biotech companies in South Korea. Under the terms of the agreement, CStone Pharmaceuticals obtained exclusive rights to lead the development and commercialization of CS5001 in all global regions outside of Korea.
Preclinical research data indicates that CS5001 demonstrates strong selective cytotoxicity in various ROR1-expressing tumor cell lines and significant in vivo anti-tumor activity in both hematological and solid tumor xenograft mouse models. Additionally, the use of targeted conjugation technology achieves a precise Drug-to-Antibody Ratio (DAR), facilitating homogeneous production and large-scale manufacturing.
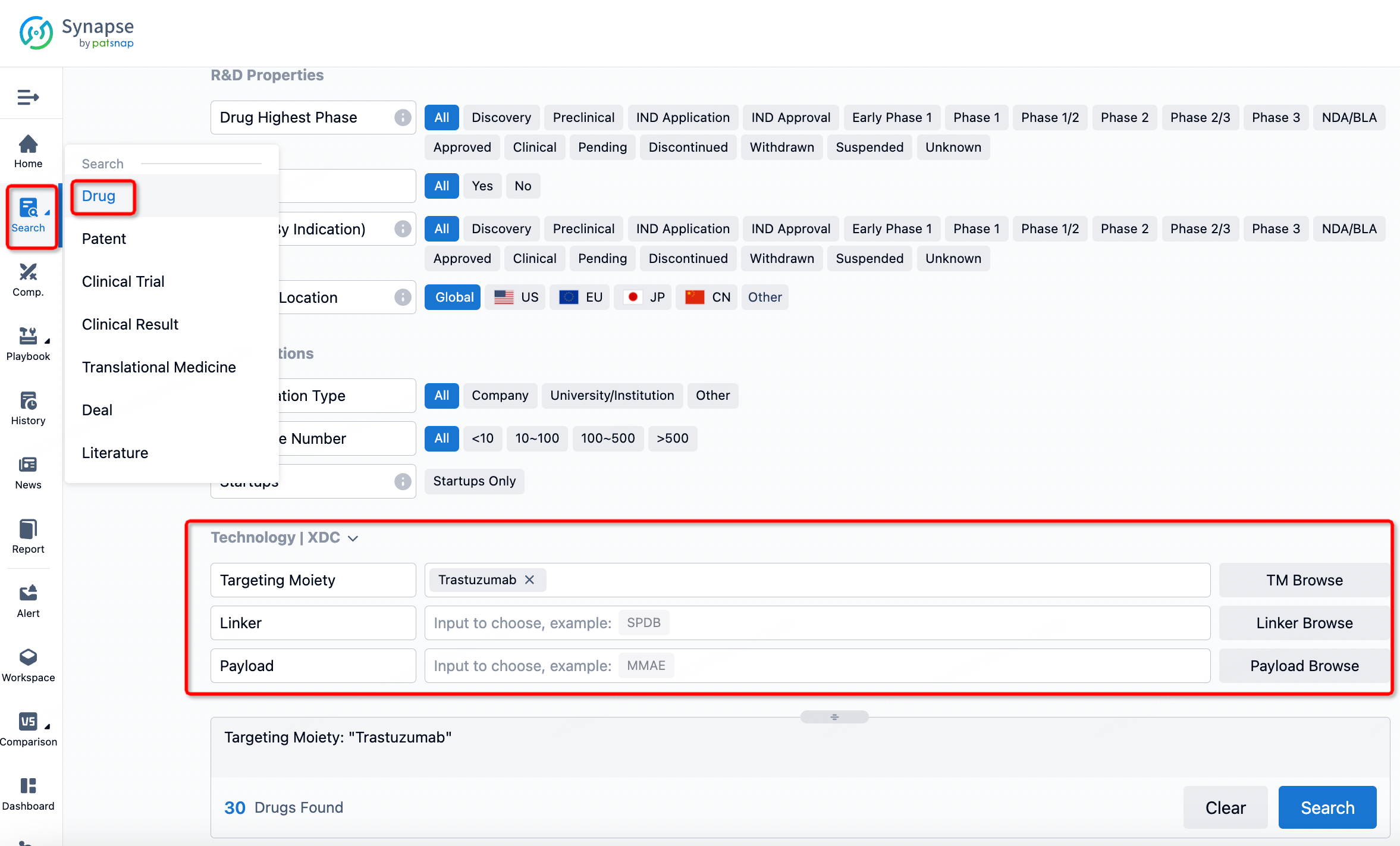
How to search for and analyze the development progress of ADC pharmaceuticals?
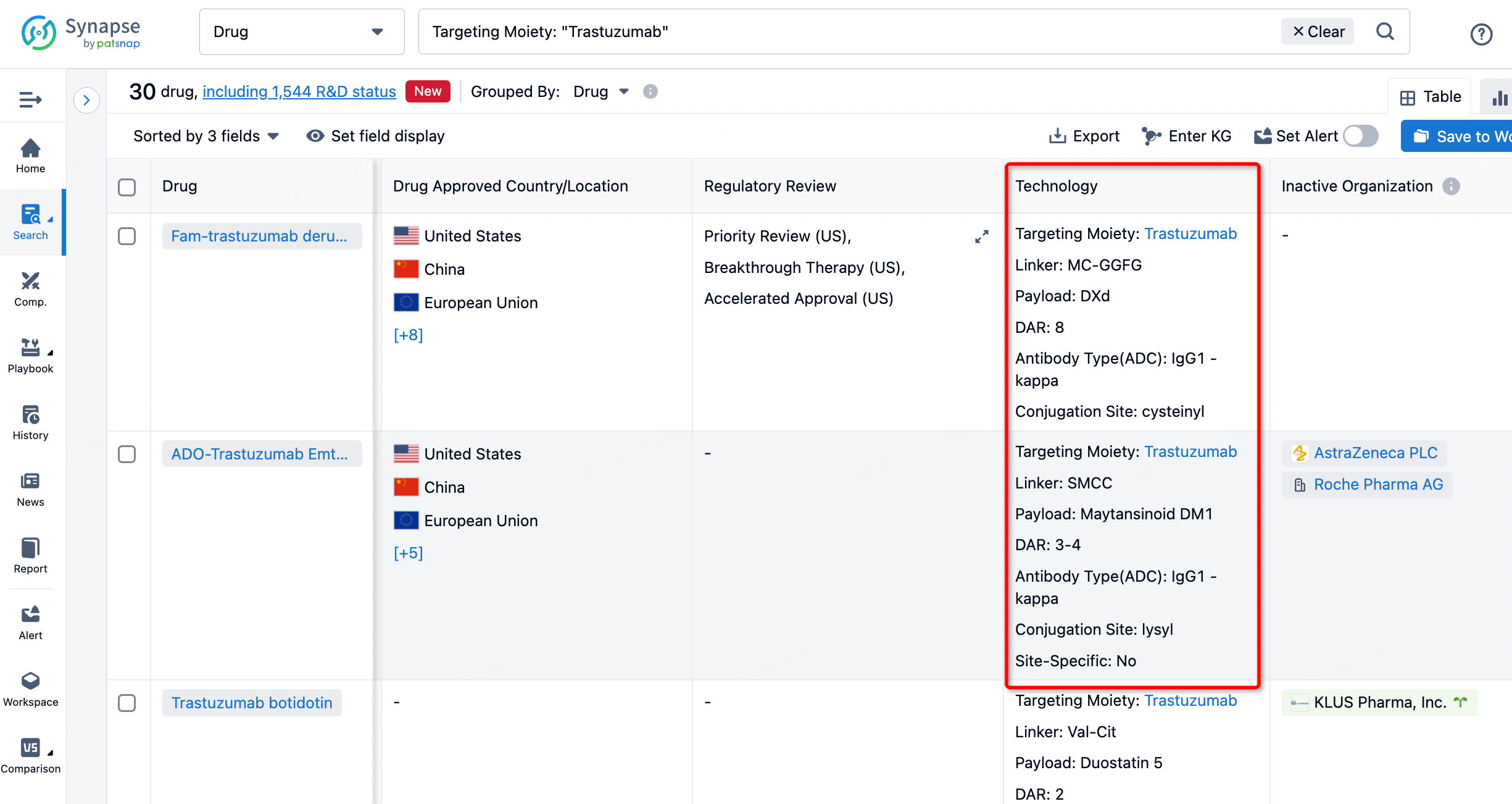
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
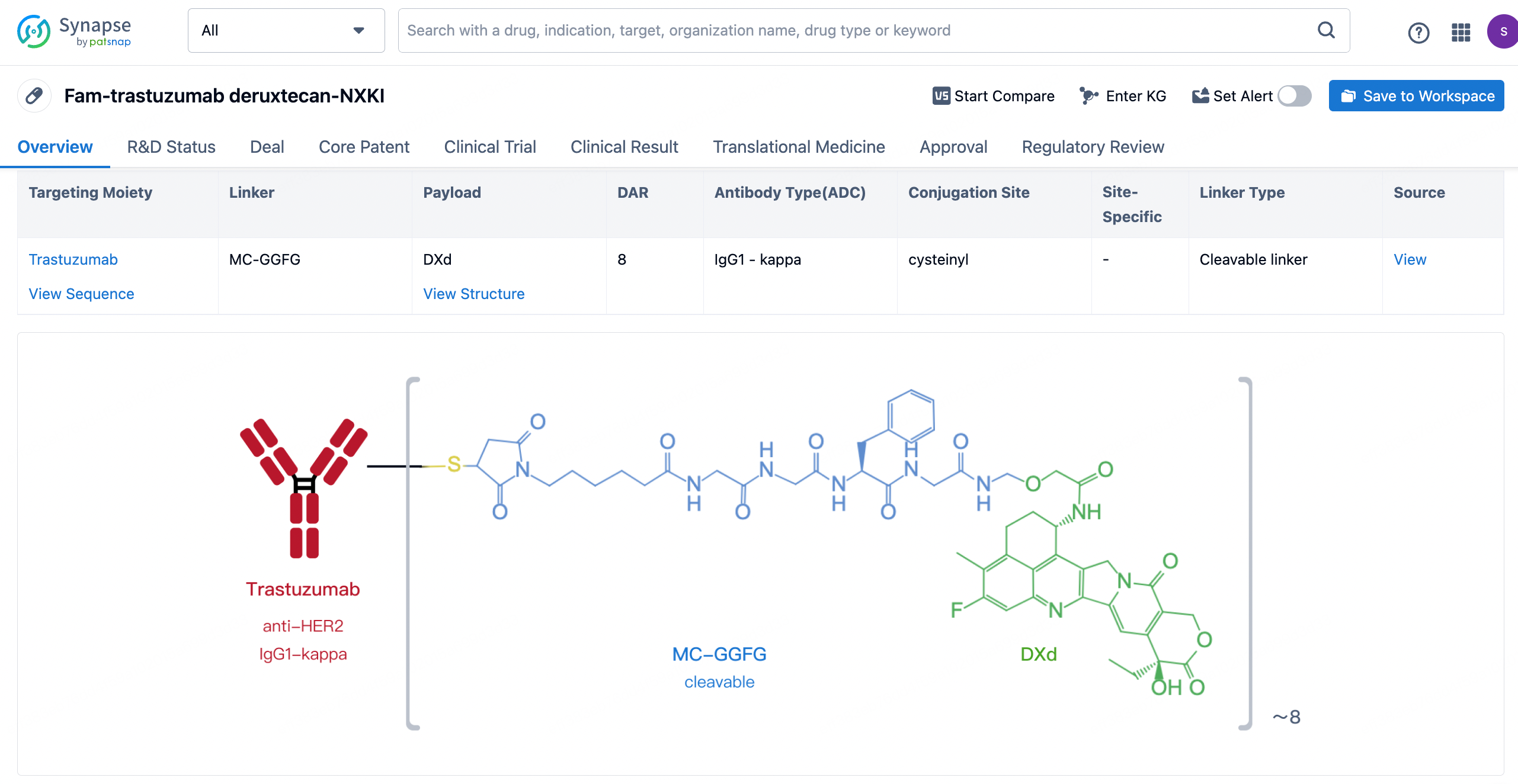
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!