What ADC-related progress will Innovent Biologics reveal at the ASCO conference?
At the upcoming 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Innovent Biologics will present a poster demonstrating the safety and efficacy of IBI343 in patients with advanced pancreatic ductal adenocarcinoma or cholangiocarcinoma.
The disclosed ADC (antibody-drug conjugate) IBI343 is a pioneering ADC molecule targeting Claudin 18.2 with potential global first-in-class attributes, leveraging Synaffix's GlycoConnect® technology for site-specific glycosylation of the cytotoxin Exatecan. This drug has demonstrated excellent Claudin 18.2-specific in vitro cytotoxic activity across various tumor cell lines with different expression levels, and robust tumor suppression activity in numerous human tumor xenograft mouse models. The site-specific glycosylation technology significantly enhances the stability of the entire ADC. In GLP toxicology studies conducted in cynomolgus monkeys, IBI343 showed favorable safety and tolerated doses as high as 30 mg/kg.
Claudin, a key protein in tight junctions of normal tissues, has four transmembrane domains and is involved in regulating paracellular permeability and electrical conductance. Claudin 18.2 is highly expressed in gastrointestinal cancers, including gastric cancer, making it a viable target for treating solid tumors such as gastric and pancreatic cancers.
IBI343, the first ADC candidate from Innovent Biologics' pipeline to enter clinical stages, targets Claudin18.2 and features site-specific glycosylation of the cytotoxin, exatecan. Upon binding to Claudin18.2-expressing tumor cells, IBI343 undergoes Claudin18.2-dependent internalization, releasing the toxic drug that causes DNA damage, leading to tumor cell apoptosis. The free toxin can also diffuse through the plasma membrane to reach and kill neighboring tumor cells, exhibiting a bystander effect.
According to the official website of the Chinese Clinical Trial Register, Innovent Biologics has initiated the global multicenter Phase 3 clinical study (G-HOPE-001) for IBI343. This study will evaluate the efficacy and safety of IBI343 as a monotherapy compared to treatments chosen by researchers, in previously treated patients with Claudin18.2-positive, HER2-negative, locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma.
On April 25th, the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) in China announced on its official website that IBI343 is considered for inclusion as a breakthrough therapy for indications in patients who have received at least two prior systemic therapies for advanced gastric/gastroesophageal junction adenocarcinoma expressing Claudin (CLDN) 18.2.
It is worth mentioning that apart from IBI343, Innovent Biologics has developed several other antibody-drug conjugates (ADCs) currently under clinical investigation. Among them, IBI334 and IBI3001 each have one study selected for the Late-Breaking Research session at the 2024 American Association for Cancer Research (AACR) Annual Meeting.
Innovent Biologics is a biopharmaceutical company dedicated to the discovery, development, and commercialization of novel drugs for oncology, autoimmune disorders, metabolic and cardiovascular diseases, ophthalmology, and other serious diseases. At this conference, the company will disclose about 20 sets of the latest clinical study data, encompassing a range of monoclonal antibodies, bispecific antibodies, and ADC candidates in development. Additionally, updates on innovative oncology drugs including Sintilimab injection, Olverembatinib, and Taletrectinib will be presented.
How to search for and analyze the development progress of ADC pharmaceuticals?
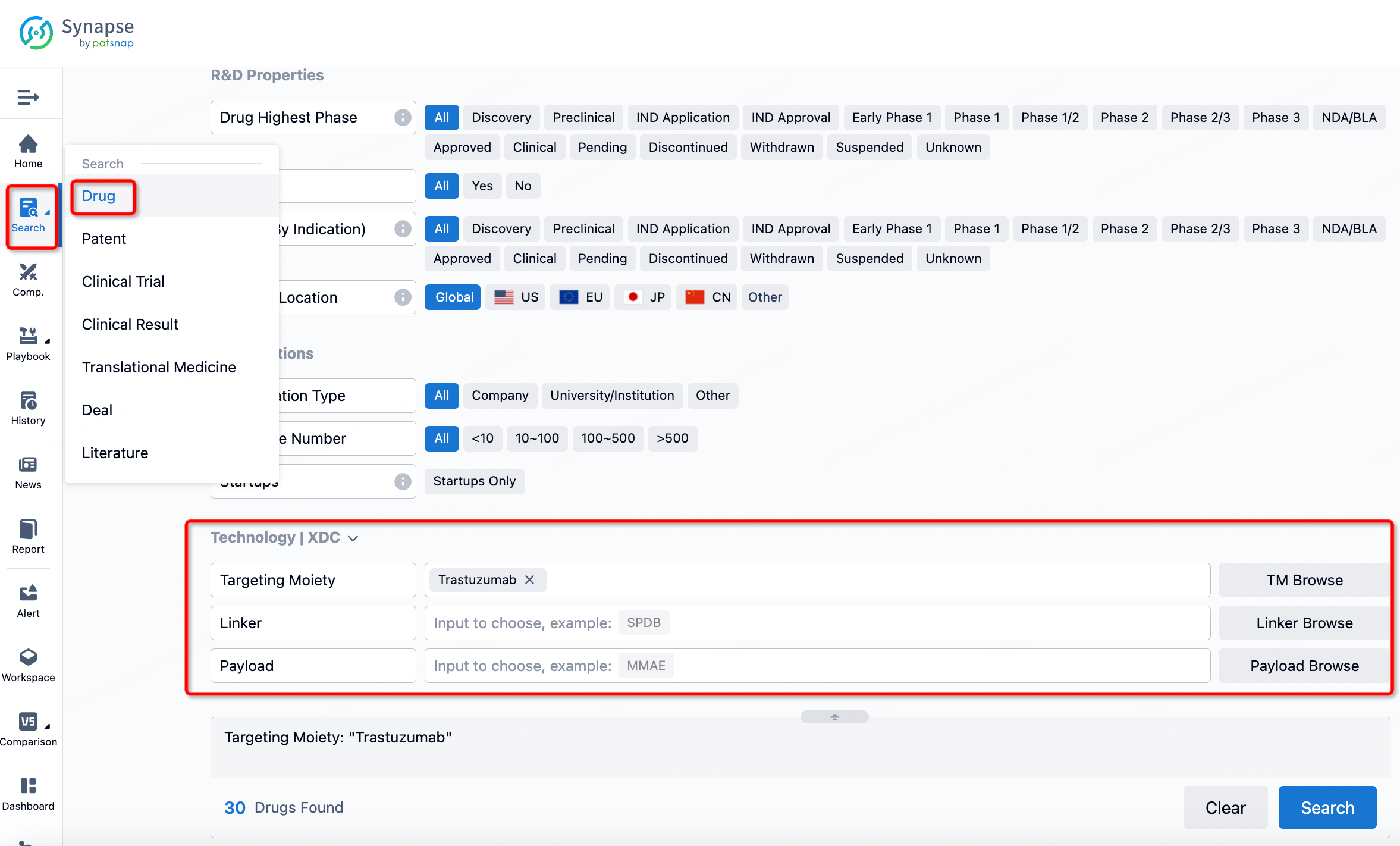
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
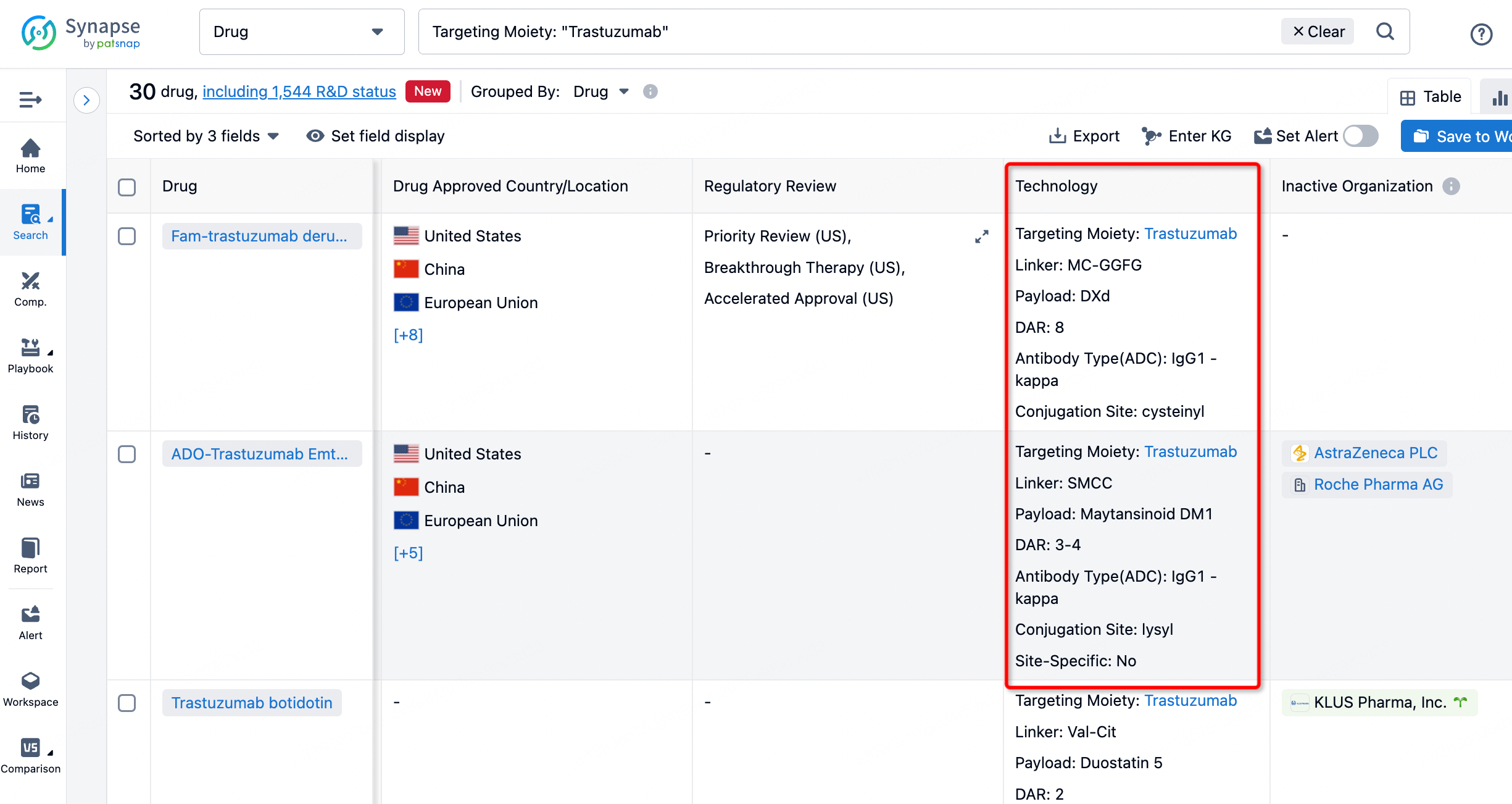
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
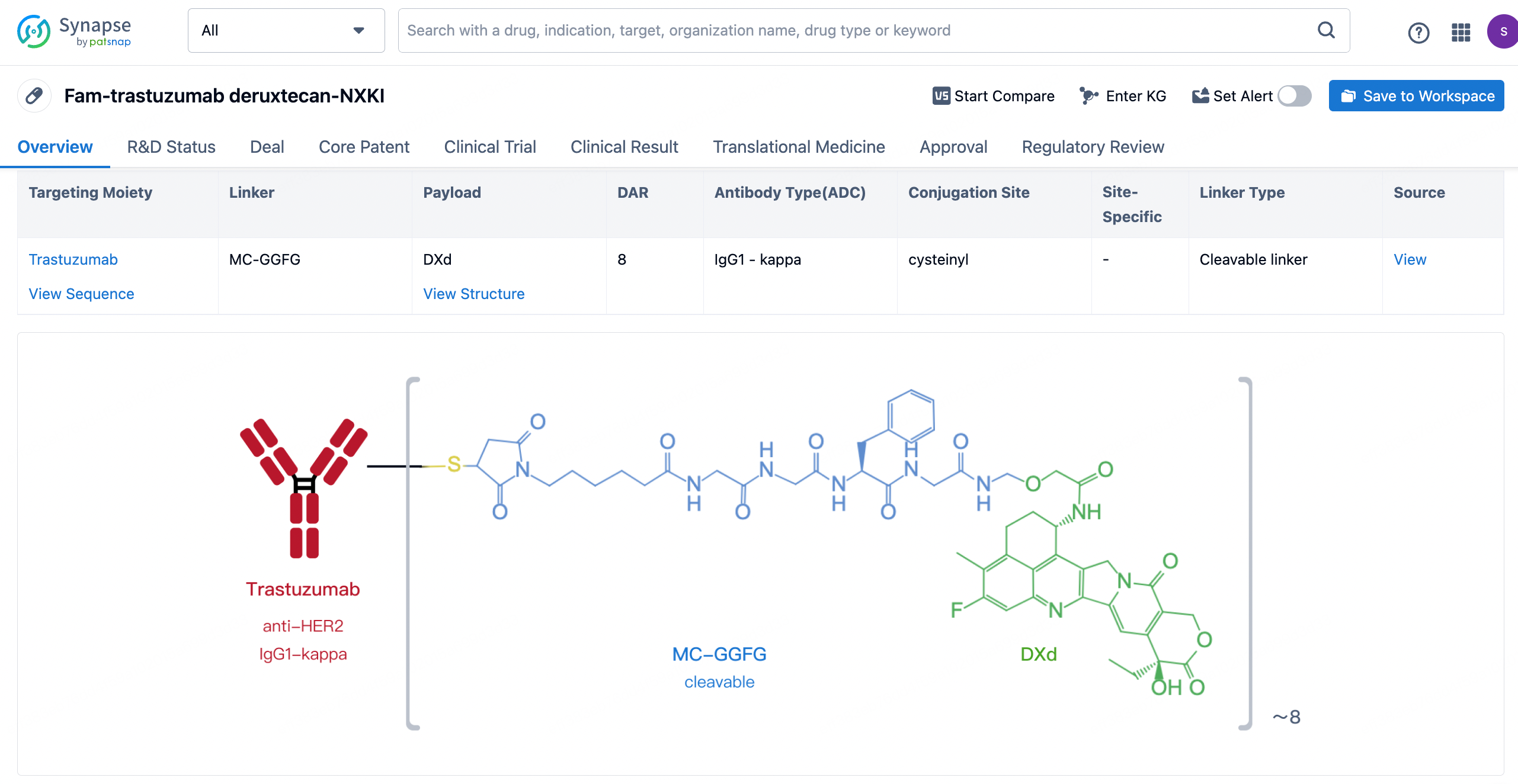
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!