What are DHODH inhibitors and how do you quickly get the latest development progress?
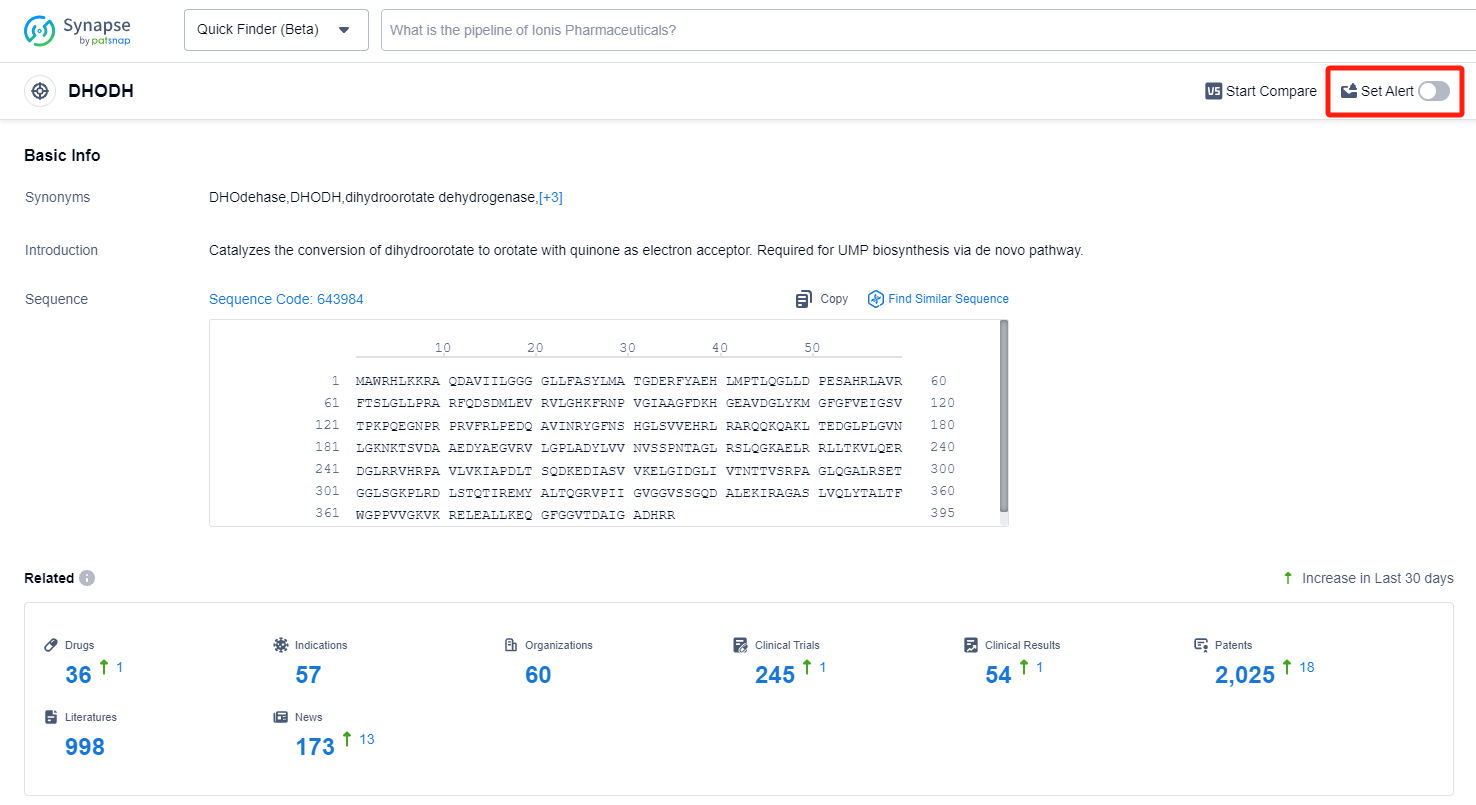
DHODH, or dihydroorotate dehydrogenase, is a kind of iron-containing flavin-dependent mitochondrial enzyme, which is a key enzyme in the catalysis of nucleic acids for pyrimidine synthesis, catalyzing the fourth step in the de novo biosynthetic pathway of pyrimidines. As an enzyme associated with the electron transfer chain, DHODH connects mitochondrial energy, cell proliferation, ROS production, and apoptosis of certain cell types. Depletion of DHODH also leads to an increase in ROS production, reduction in membrane potential, and slow cell growth. Blocking the DHODH synthesis pathway can effectively inhibit the proliferation of activated immune cells and the secretion of cytokines, thereby suppressing cytokine storms caused by diseases. At the same time, inhibiting DHODH activity significantly reduces nucleoside raw materials, hinders viral nucleic acid synthesis, inhibits viral replication, has broad-spectrum antiviral activity, and is not easy to cause drug-resistant mutations. DHODH is a validated therapeutic target that can be used to treat autoimmune diseases, including rheumatoid arthritis and multiple sclerosis. Now the field of drug development for DHODH inhibitors is full of new opportunities.
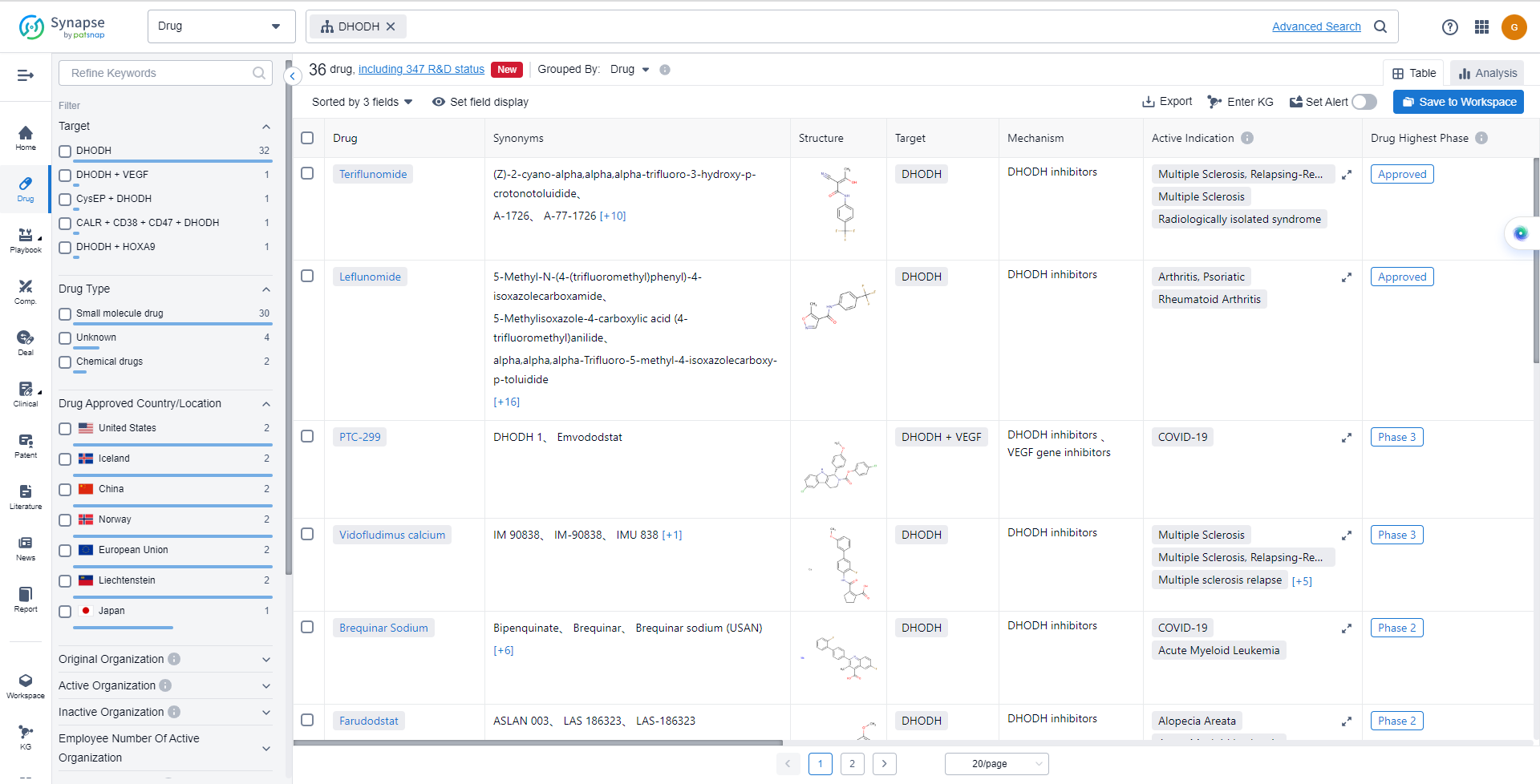
Leflunomide is the first FDA-approved DHODH inhibitor. It is a synthetic isoxazole compound developed by Sanofi. In 1998, the FDA approved the market launch application for leflunomide for the treatment of rheumatoid arthritis, lupus nephritis, and psoriatic arthritis. The listing of leflunomide is based on the Phase III US301 trial. The research results show that leflunomide has comparable clinical efficacy and tolerability with methotrexate and sulfasalazine, and has better efficacy and tolerability compared with placebo. Among 482 patients initially treated, the response rate reached 52%.
Teriflunomide is also a DHODH inhibitor developed by Sanofi. In 2012, the FDA approved its market launch. Teriflunomide inhibits the rapid differentiation of T cells, thereby slowing the progression of multiple sclerosis. Based on the global phase III clinical trial TEMSO study with thousands of cases, teriflunomide reduced the annual relapse rate of multiple sclerosis by 31% compared to placebo. In addition, teriflunomide also has antiviral effects on several viruses, including CMV, HSV1, and BK virus. It inhibits viral replication by interfering with the formation of nucleocapsids and virus particle assembly.
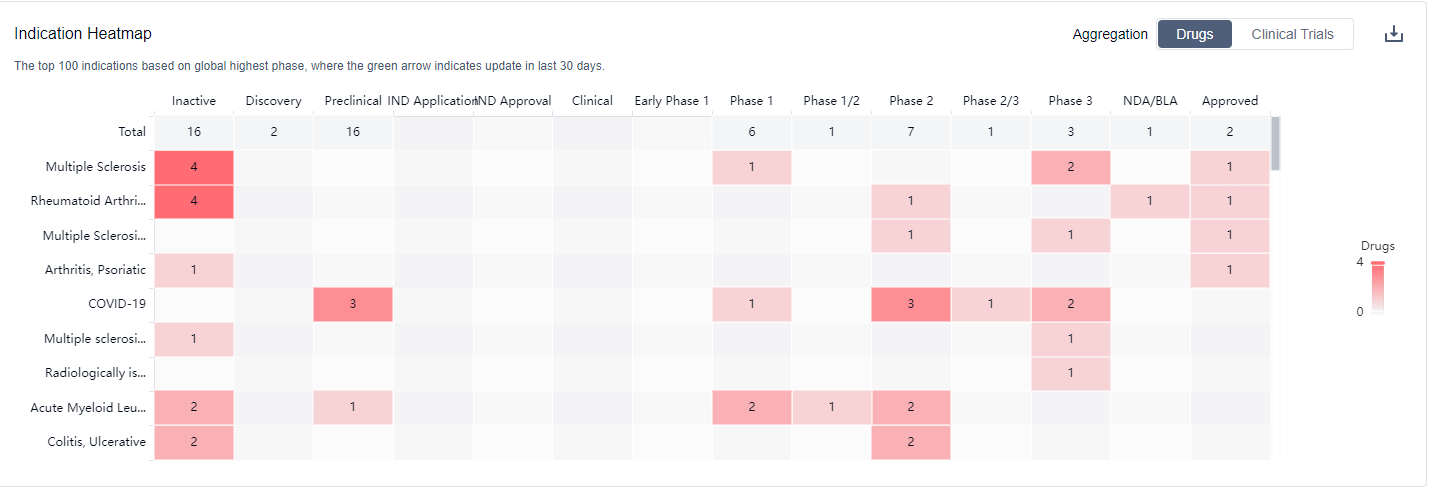
The analysis of the target DHODH reveals a competitive landscape with multiple companies actively involved in the research and development of drugs. Sanofi, Immunic, Inc., PTC Therapeutics, Inc., and Aslan Pharmaceuticals Ltd. are among the companies with the highest number of drugs in various stages of development. The indications for DHODH-targeting drugs cover a wide range of diseases and conditions, indicating their potential therapeutic applications. Small molecule drugs and unknown drugs are progressing rapidly, suggesting ongoing efforts to develop innovative compounds and identify new drug candidates. The United States, European Union, China, and other countries have made significant progress in the development of DHODH-targeting drugs, indicating a global interest in this target. Overall, the target DHODH presents a promising opportunity for the pharmaceutical industry, with potential for further growth and development in the future.
The mechanism of action of DHODH inhibitors
DHODH inhibitors are a type of drugs that target and inhibit the enzyme dihydroorotate dehydrogenase (DHODH). DHODH is an enzyme involved in the de novo pyrimidine biosynthesis pathway, which is essential for the production of DNA and RNA in cells. By inhibiting DHODH, these inhibitors disrupt the synthesis of pyrimidine nucleotides, which are crucial for cell proliferation and growth.
From a biomedical perspective, DHODH inhibitors have shown potential in the treatment of various diseases, including cancer, autoimmune disorders, and viral infections. In cancer, these inhibitors can inhibit the growth of tumor cells by blocking their ability to produce DNA and RNA, thereby impeding their proliferation. In autoimmune disorders, DHODH inhibitors can modulate the immune response by suppressing the proliferation of immune cells involved in the disease process. Additionally, DHODH inhibitors have been investigated as antiviral agents, particularly against RNA viruses, by interfering with their replication and propagation.
Overall, DHODH inhibitors represent a class of drugs that target an important enzyme involved in nucleotide synthesis, making them potential candidates for therapeutic interventions in various disease conditions.
List of DHODH Inhibitors
The currently marketed DHODH inhibitors include:
- Teriflunomide
- Leflunomide
- PTC-299
- Vidofludimus calcium
- Brequinar Sodium
- Farudodstat
- KIO-101
- RP-7214
- Teriflunomide Sodium
- Vidofludimus
For more information, please click on the image below.
What are DHODH inhibitors used for?
Currently, in clinical practice, DHODH inhibitors are mainly used for autoimmune diseases such as Rheumatoid Arthritis, Lupus Nephritis, and Multiple Sclerosis. Multiple in vitro and clinical studies have shown that drugs that inhibit DHODH have anti-inflammatory, anti-tumor, anti-malaria parasite, and antiviral effects. For more information, please click on the image below to log in and search.
How to acquire the most recent advancement in DHODH inhibitors?
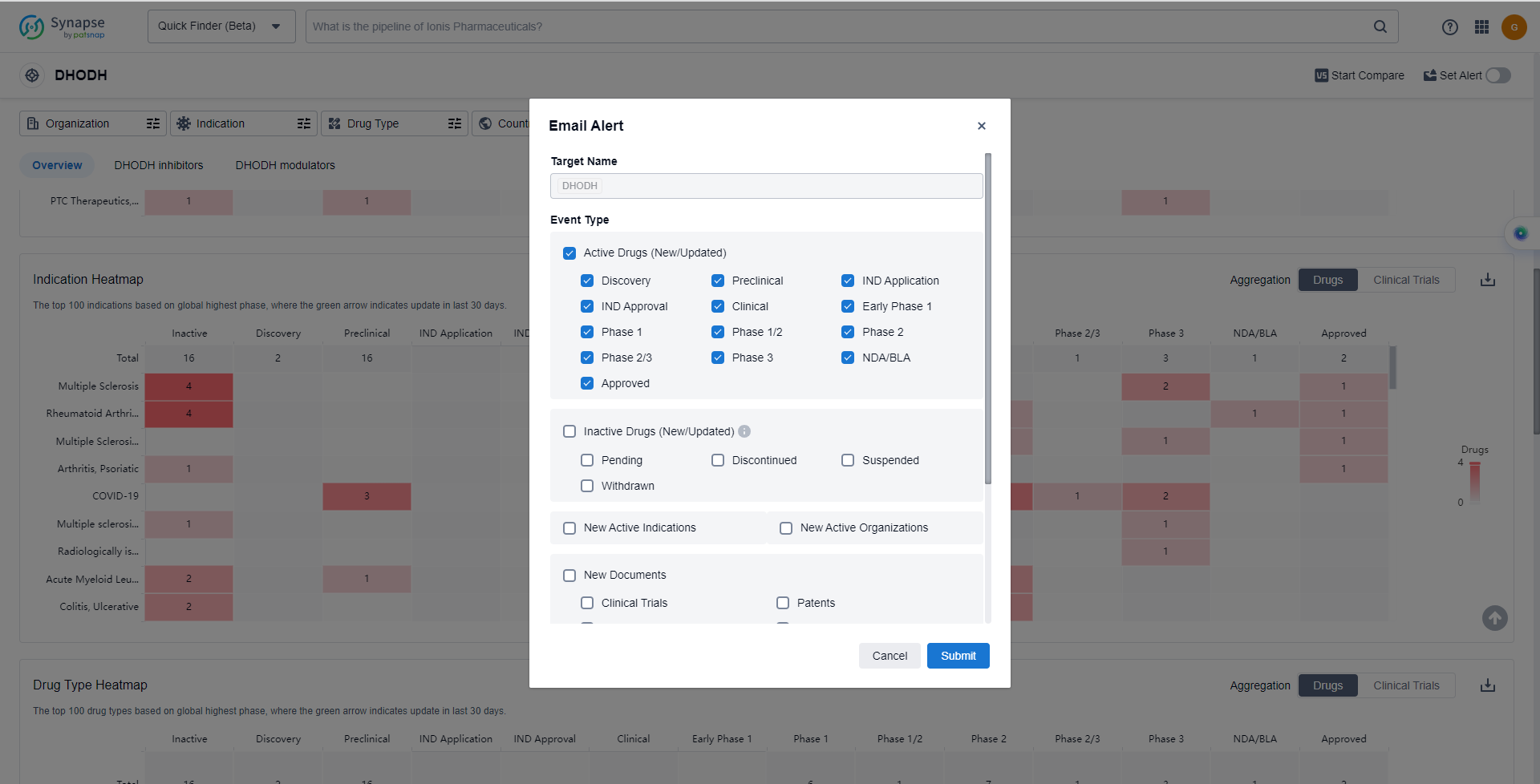
In the Synapse database, you can keep abreast of the latest research and development advances of DHODH inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!