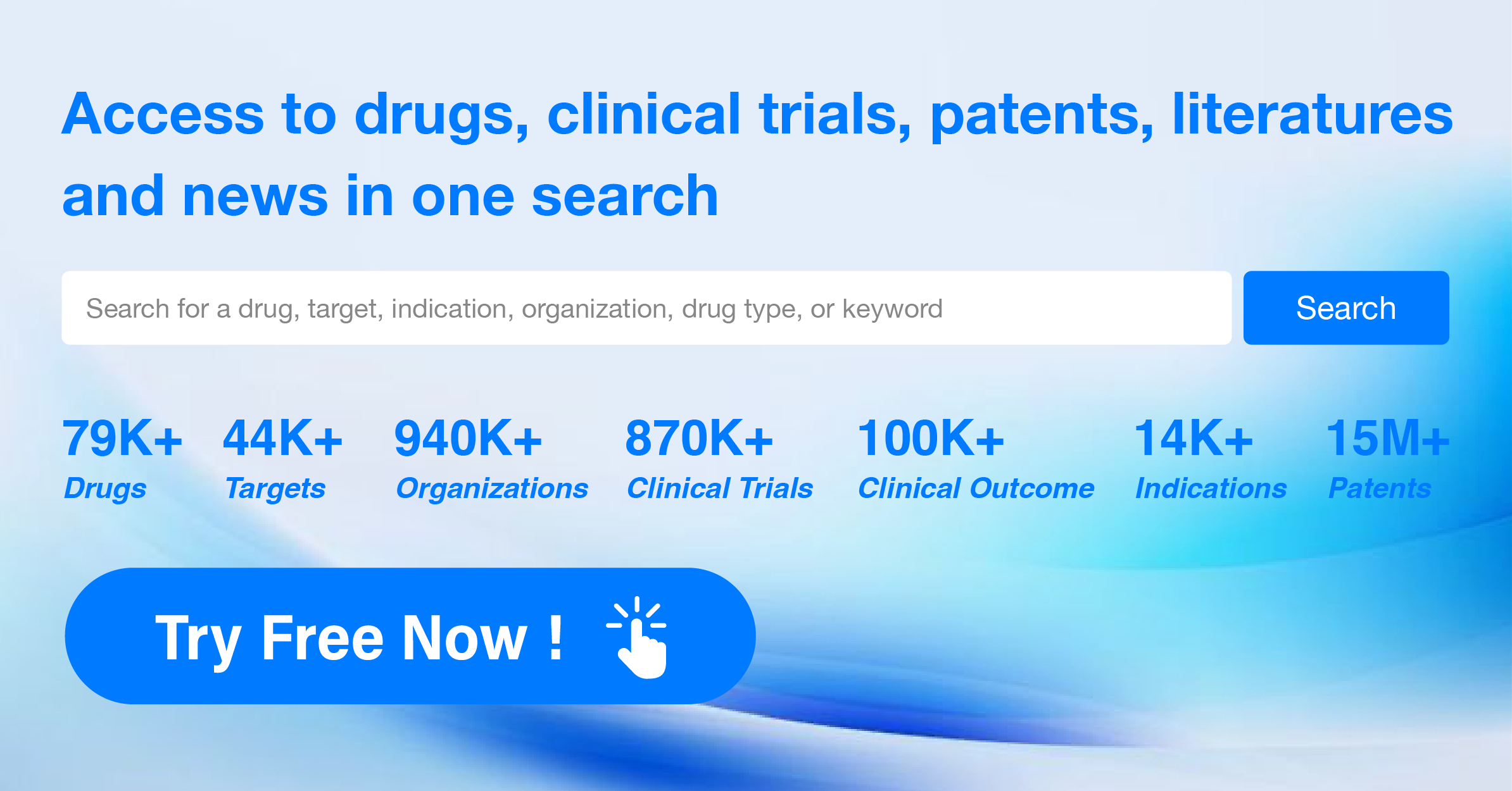
What is post marketing research?
According to pharmacoepidemiology: post marketing research of drugs refers to the research on the use and effect of drugs following a specific research scheme after they are approved for marketing. Post marketing research of drugs includes clinical trials, investigations (such as observational epidemiological studies, post marketing clinical safety re evaluation, etc.) and laboratory studies, which are usually divided into two parts: post marketing requirements (PMR) and post marketing commitments (PMC).
(1) Evaluate the benefits and risks of drug use in general or special populations
(2) evaluate the effectiveness of drugs under conditions of widespread use
(3) evaluate the known serious risks related to drug use
(4) evaluate the serious risk signals related to drug use
(5) find potential unexpected serious risks
(6) Improve the pre marketing effectiveness data of drugs
(7) improve the clinical pharmacology information (food and drug administration.