Moberg Pharma Reveals Phase 3 Trial Results and Reclaims EU Rights to MOB-015
Moberg Pharma AB (OMX: MOB) has reported that MOB-015 (topical terbinafine) failed to achieve the primary endpoint in its phase 3 trial, which involved 8 weeks of daily administration followed by maintenance doses on a weekly basis. The company will now concentrate on the successful daily dosing regimen that has received approval in 13 EU nations.
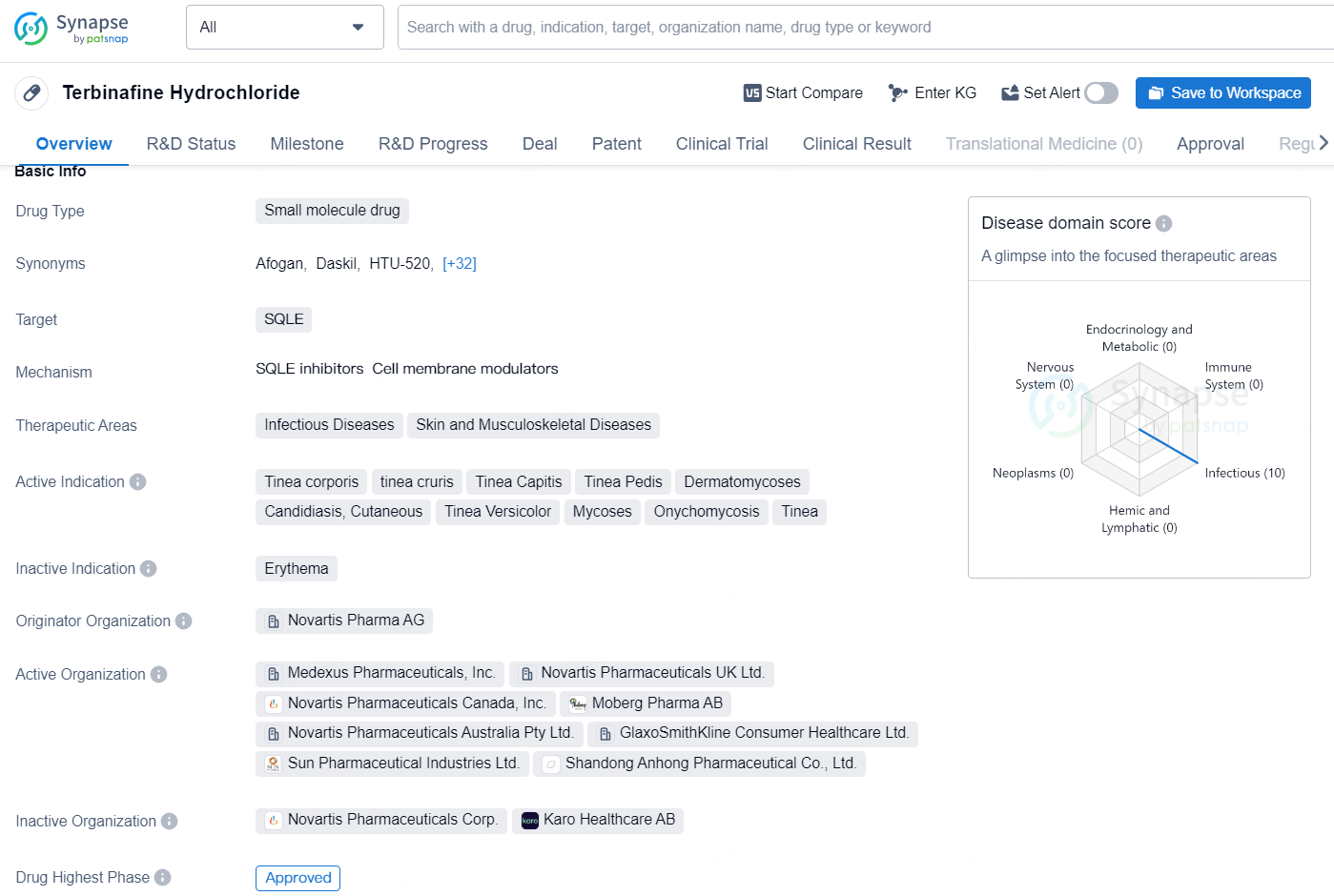
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In accordance with earlier discussions from September 13, 2024, the findings now indicate that the primary endpoint has not been achieved. The Phase 3 clinical trial in North America involved 33 research sites across the US and Canada and included 384 participants, consisting of 260 who received MOB-015 and 124 who were given a placebo. This trial differed from previous investigations of MOB-015, which served as the foundation for the drug’s approval in 13 EU nations, by implementing a reduced dosage protocol—8 weeks of daily administration followed by weekly maintenance for 40 weeks, as opposed to continuous daily treatment for the entire duration.
The North American trial was anticipated to further substantiate the product's claims. A favorable result would have provided a competitive edge by only necessitating weekly treatments post-initial phase. The outcomes indicate that an 8-week daily treatment is inadequate for effective management of onychomycosis, necessitating a longer duration of daily therapy, as endorsed in the EU.
Following a comprehensive evaluation of its project pipeline, Bayer Consumer Health has opted to halt the forthcoming launch of MOB-015 due to strategic considerations and the topline results obtained. Consequently, Bayer and Moberg Pharma have mutually agreed to terminate their licensing arrangement, allowing Moberg Pharma to reclaim full rights to MOB-015 within the EU, alongside maintaining the milestone payments already made by Bayer.
We continue to believe in the competitive advantages of MOB-015, evidenced by its recent successful launch in Sweden, where it is marketed as Terclara® and holds a leading market position with a 44% market growth. The drug's mycological cure rate of 76% with daily dosing is exceptional for a topical treatment of onychomycosis, and the success in Sweden validates that the marketing approach and product claims resonate well with consumers.
Our strategic plan has been to integrate direct sales in the U.S. market while pursuing strategic partnerships in other key regions. Given the trial results, we are reevaluating our strategies for the U.S. and redirecting our attention towards Europe, which offers significant growth potential. Moberg Pharma aims to enhance its involvement in the value chain within Europe by taking a proactive role in commercialization and establishing a stronger direct presence, including brand ownership. To facilitate this approach, Moberg Pharma is currently in talks with potential European partners to determine the best course of action moving forward.
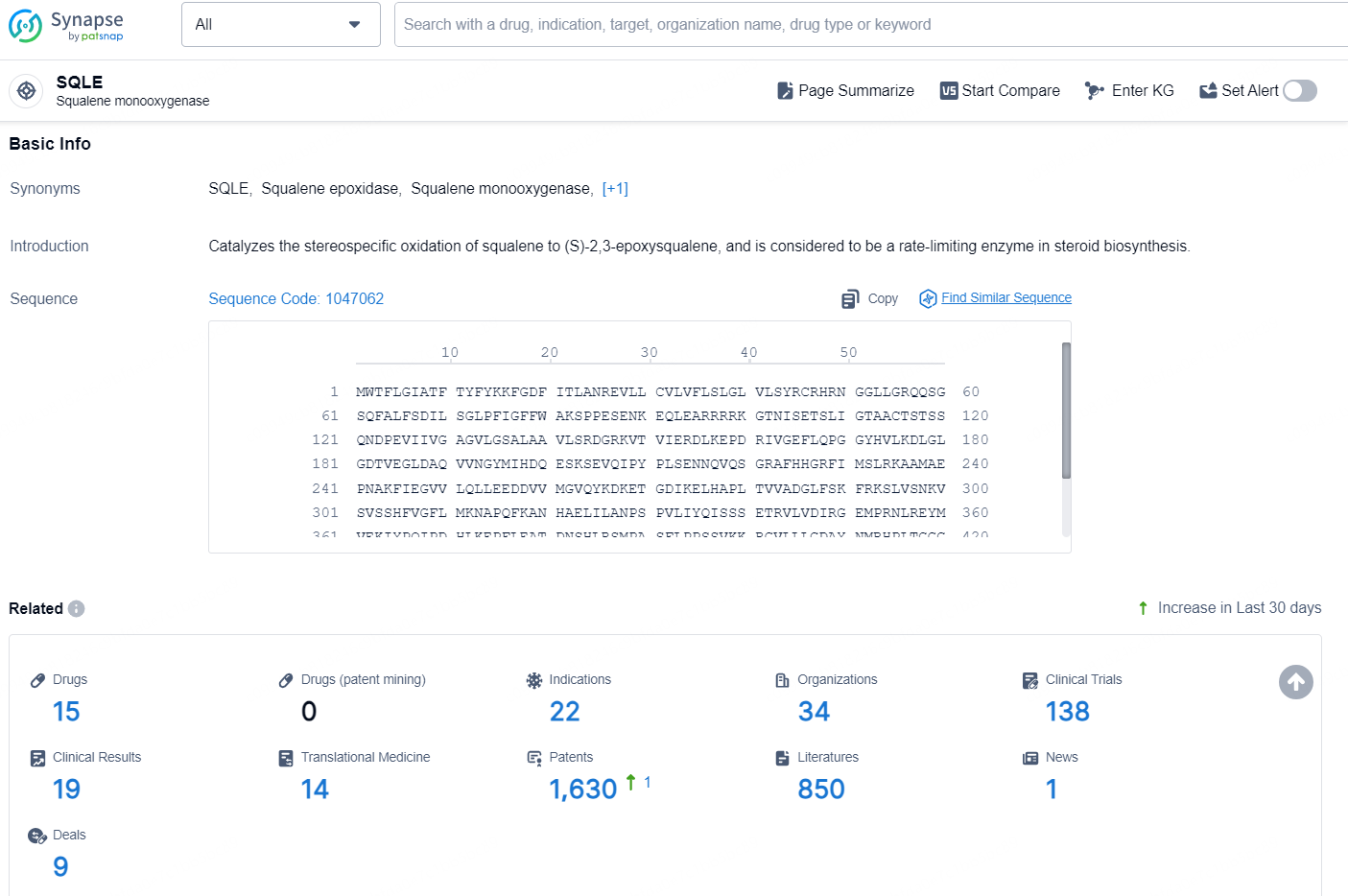
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of December 17, 2024, there are 15 investigational drugs for the SQLE target, including 22 indications, 34 R&D institutions involved, with related clinical trials reaching 138, and as many as 1630 patents.
Terbinafine Hydrochloride is a small molecule drug that targets SQLE and is used to treat a range of infectious and skin/musculoskeletal diseases. The drug is approved for a variety of conditions including Tinea corporis, tinea cruris, Tinea Capitis, Tinea Pedis, Dermatomycoses, Candidiasis, Cutaneous, Tinea Versicolor, Mycoses, Onychomycosis, and Tinea.