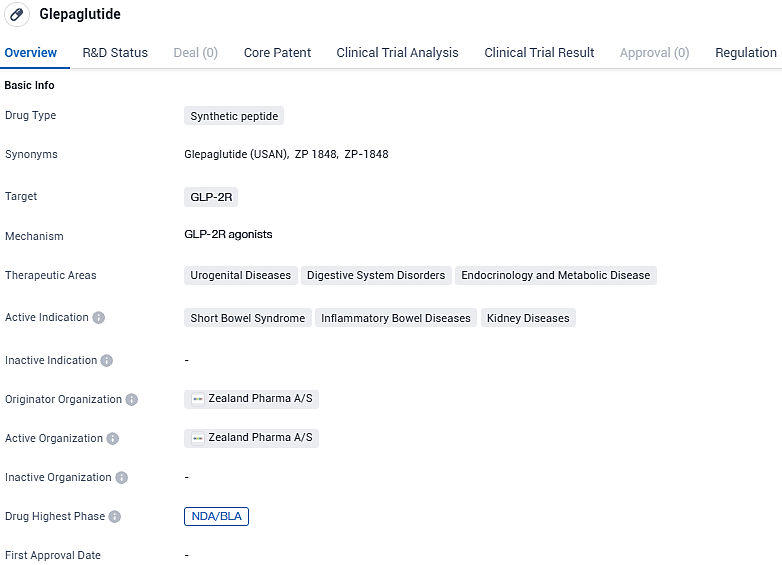
Zealand Pharma submits FDA approval request for glepaglutide to treat short bowel syndrome
Zealand Pharma A/S, a corporation that specializes in pioneering new peptide therapies, has officially reported the filing of a New Drug Application with the US Food and Drug Administration regarding glepaglutide. This extended-release version of the GLP-2 analogue is designed to manage short bowel syndrome in adults who require intravenous nutritional support.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"Patients grappling with a severe and chronic form of Short bowel syndrome accompanied by intestinal failure typically rely on intravenous means for hydration and nutrition," stated Zealand Pharma's Chief Medical Officer, David Kendall, MD. "Although vital for survival, this mode of parenteral nutrition substantially limits everyday activities and brings a considerable risk of severe, potentially fatal complications."
Dr. Kendall expressed optimism about glepaglutide, emphasizing its promise to ease the frequency of administration and the overall demands of parenteral support on those battling with Short bowel syndrome and intestinal failure. He announced the company's enthusiasm in submitting glepaglutide for regulatory consideration and hopes for its approval in the United States.
This submission leverages data from the critical EASE-1 Phase 3 trial, augmented by preliminary findings from two active follow-up trials, EASE-2 and EASE-3, as well as insights from a focused EASE-4 mechanistic study.
The EASE-1 study, a masked, placebo-controlled Phase 3 trial, scrutinized the effects and safety profile of glepaglutide administered once or twice per week via subcutaneous injections at a 10 mg dosage. The trial incorporated 106 participants with Short bowel syndrome and intestinal failure who required parenteral feeding a minimum of three days each week.
Building on the initial protocol, EASE-2 continues as a blinded study where patients originally partook in EASE-1 offerings comprised of glepaglutide treatments at a 10 mg dosage, once or twice weekly. Those who were on a placebo during EASE-1 were switched to a determined regimen of glepaglutide.
EASE-3 focuses on the feasibility of administering glepaglutide weekly with an auto-injector, with an interim assessment on the first 43 individuals transitioning from EASE 2, demonstrating a sustained reduction in necessary parenteral support. Lastly, EASE-4 is a 3b Phase trial, dedicated to understanding glepaglutide's impact on nutrient and fluid absorption in the intestines.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
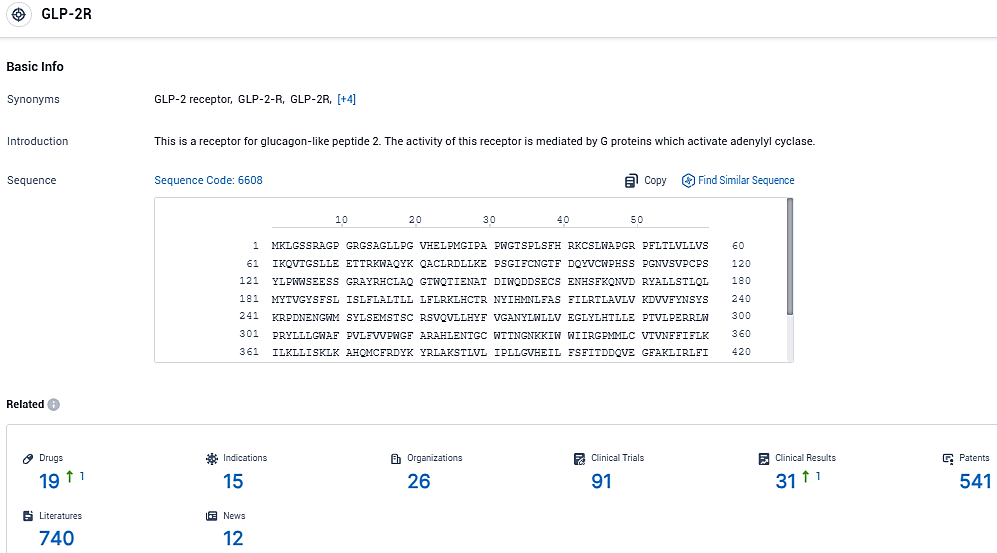
According to the data provided by the Synapse Database, As of December 30, 2023, there are 19 investigational drugs for the GLP-2R target, including 15 indications, 26 R&D institutions involved, with related clinical trials reaching 91, and as many as 541 patents.
Glepaglutide is a long-acting GLP-2 analog in development as a potential treatment option for short bowel syndrome. Glepaglutide is being developed as a liquid product in an autoinjector designed for subcutaneous administration by twice weekly dosing, aimed to reduce, or eliminate, the need for parenteral support in people living with SBS. The U.S. Food and Drug Administration has granted orphan drug designation for glepaglutide for the treatment of SBS.