Last update 19 Sep 2024
Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)
Last update 19 Sep 2024
Overview
Basic Info
Drug Type Vaccine |
Synonyms Autologous dendritic cellderived exosomes, DEX |
Target- |
Mechanism Immunostimulants |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
Drug Highest PhaseDiscontinuedPhase 2 |
First Approval Date- |
Regulation- |
Related
1
Clinical Trials associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)Nebulized Midazolam, Dexmedetomidine, and Their Combination in Sedation of Preschoolers Undergoing Dental Treatment: A Randomized Clinical Trial
The aim of the study is to evaluate the effect of nebulized Midazolam, Dexmedetomidine, and their combination as procedural, moderate sedative agents in preschoolers undergoing dental treatment.
Start Date27 Nov 2017 |
Sponsor / Collaborator |
100 Clinical Results associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)
Login to view more data
100 Translational Medicine associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)
Login to view more data
100 Patents (Medical) associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)
Login to view more data
10,471
Literatures (Medical) associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)31 Dec 2024·OncoImmunology
Trial watch: dexmedetomidine in cancer therapy
Review
Author: Carnet Le Provost, Killian ; Kepp, Oliver ; Kroemer, Guido ; Bezu, Lucillia
31 Dec 2024·Animal Cells and Systems
Role of Carbohydrate response element-binding protein in mediating dexamethasone-induced glucose transporter 5 expression in Caco-2BBE cells and during the developmental phase in mice
Article
Author: Kim, Jaewan ; Oh, Ah-Reum ; Lee, Ho-Jae ; Hwang, Soonjae ; Park, Sangbin ; Cha, Ji-Young
31 Dec 2024·Hematology
Honokiol induces apoptosis and autophagy in dexamethasone-resistant T-acute lymphoblastic leukemia CEM-C1 cells
Article
Author: Tan, Yajuan ; Zhang, Suqian ; Liu, Yang
11
News (Medical) associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)30 Dec 2022
FinancialNewsMedia.com News Commentary
Palm Beach, Fla., Dec. 30, 2022 /PRNewswire/ -- According to the Centers for Disease Control and Prevention, arthritis affects approximately one in every four Americans, or 58.5 million people. The global rheumatoid arthritis drugs market is expected to benefit significantly from increased launches of innovative biologics and expanding generic medication penetration. The growth of global rheumatoid arthritis drugs market is also being driven by growing constant efforts for development of rheumatoid arthritis drugs and medications by government agencies. The government is also taking assistance of major market players for development and growth of global rheumatoid arthritis drugs market. In addition, government agencies are also investing in innovative and latest technologies for the development of rheumatoid arthritis drugs in the global market. As a result, all of these aforementioned factors are boosting the growth of global rheumatoid arthritis drugs market. A report from Precedence Research projected that the global rheumatoid arthritis drugs market, which was estimated at US$ 60.02 billion in 2021 is expected to hit US$ 70 billion by 2030 with a registered CAGR of 1.72% from 2022 to 2030. The report said: "One of the significant factors driving the growth of global rheumatoid arthritis drugs market is rising prevalence of rheumatoid arthritis… As a result, more rheumatoid arthritis drugs will be adopted, propelling the rheumatoid arthritis drugs market forward… Another factor driving the growth of global rheumatoid arthritis drugs market is growing patient awareness regarding rheumatoid arthritis disorders… there is a movement in the rheumatoid arthritis drugs market toward combination medicines that deliver better benefits for patients." Active biotechs in the markets today include:
Silo Pharma, Inc. (NASDAQ: SILO),
Baudax Bio, Inc. (NASDAQ: BXRX),
Hoth Therapeutics, Inc. (NASDAQ: HOTH),
Kala Pharmaceuticals, Inc. (NASDAQ: KALA),
Nuwellis, Inc. (NASDAQ: NUWE).
Precedence Research continued: "The biopharmaceutical production capacity is expected to increase as manufacturing capabilities are improved. In the synthesis and processing of biopharmaceuticals, single use methods are also being used. In addition, researchers are looking towards more productive species and expression methods. Revenue generation is expected to be fueled by the development of reagents and cell lines that improve biological products output. North America region is the fastest growing region in the rheumatoid arthritis drugs market. The U.S. hold the highest market share in the North America rheumatoid arthritis drugs market… In addition, the Canadian government estimates that 374,000 Canadians aged 16 and up suffer from rheumatoid arthritis. Because of the disease's extensive prevalence, more medicines will be taken, leading in a growth in the rheumatoid arthritis drugs market in North America."
Silo Pharma, Inc. (NASDAQ: SILO)
BREAKING NEWS:
Silo Pharma Announces Positive Study Results of SPU- 21 for Arthritis - SPU-21 effective in controlling arthritis progression - SPU-21 Demonstrates Positive Data in Arthritis Suppression using Silo's Novel Joint Homing Peptide -- Silo Pharma, Inc. ("the Company"), a developmental stage biopharmaceutical company focused on merging traditional therapeutics with psychedelic research, today announced positive interim data from its dose optimization study of SPU-21 joint homing peptides for subcutaneous administration of anti-arthritic agents. Silo Pharma is pursuing a development plan utilizing its liposomal joint homing peptides as a potential therapy for rheumatoid arthritis (RA).
In the most recent phase of this ongoing animal study, tests were conducted to evaluate the disease-suppressive effects of an SPU-21 peptide-guided anti-arthritis drug versus the drug alone. The drug used in the study was dexamethasone (DEX), a corticosteroid used for its anti-inflammatory and immunosuppressant effects. Earlier results of the same study successfully demonstrated that the subcutaneous (SC) route of liposomal administration (small needle injection into shallow soft tissue just under the layer of skin) is well-suited for use in targeted drug delivery of anti-arthritic agents.
"The positive results of these latest tests show that our peptide with DEX given subcutaneously was effective in controlling arthritis progression. The effect of lipo-DEX was superior to that of DEX alone when both were administered via the SC route," said Eric Weisblum, Chief Executive Officer of Silo Pharma. "Since patients widely prefer SC administration over intravenous (IV) infusion for multiple reasons, we believe the superiority and practicality of our liposomal joint homing peptide bode well for broad market potential. Meanwhile, we continue to explore other novel therapeutics for optimal pairing with SPU-21, targeting rheumatoid arthritis as our initial indication."
Silo Pharma is advancing the development of SPU-21 liposomal joint homing peptides in collaboration with the University of Maryland, Baltimore (UMB).
CONTINUED…
Read this full release for
Silo Pharma
at:
Other recent biotech developments in the markets include:
Baudax Bio, Inc. (NASDAQ: BXRX) recently announced the initiation of a clinical study evaluating the safety, tolerability profile, and intubation conditions of BX1000 for neuromuscular blockade (NMB) in patients undergoing elective surgery.
This randomized, double-blind clinical trial will study BX1000 in approximately 80 adult patients, 18-65 years of age, who undergo elective surgery utilizing total intravenous anesthesia (TIVA) in an outpatient setting. Patients will undergo elective surgery with an intravenous (IV) line for anesthesia and study drug administration. Once anesthetized, neuromuscular monitoring will be initiated via electromyography (EMG), and approximately 3-5 minutes after induction of anesthesia, the randomized NMB treatment will be administered as an IV bolus. Intubation conditions will be assessed at 60 seconds after administration of the NMB dose and will be reassessed at 90 and 120 seconds if needed, with tracheal intubation performed when clinically acceptable conditions are identified. These "intubating conditions" represent the endpoint for NDA approval for NMB agents. Following successful tracheal intubation, patients will proceed to undergo their elective surgical procedures according to the standard practice of the investigator or surgical unit. Patients will be monitored post-surgery in the anesthesia recovery area and will be transferred to the inpatient facility where they will remain for at least 8 hours following NMB administration, to be discharged at the discretion of the investigator. There will be an in-person follow-up visit and several telephonic safety follow ups as well.
Hoth Therapeutics, Inc. (NASDAQ: HOTH), a patient-focused biopharmaceutical company, recently announced that the U.S. Food and Drug Administration (FDA) has accepted an Investigational New Drug (IND) application for the company's HT-001 therapeutic for the treatment for rash and skin disorders associated with epidermal growth factor receptor (EGFR) inhibitor therapy. EGFR inhibitors are critical therapeutic agents for the treatment of non-small cell lung cancer (NSCLC), pancreatic cancer, colorectal cancer, squamous-cell carcinoma of the head and neck, and breast cancer.
"We are excited to begin our trial and bring hope to patients who are suffering. With no specific treatment currently approved for the treatment of skin toxicities associated with EGFRi therapies, this trial brings us one step closer to a new treatment option for underserved cancer patients," said Robb Knie, CEO of Hoth Therapeutics, Inc. "We look forward to advancing HT-001 into the clinical phase as we believe that this novel therapeutic will be a key treatment in the onco-dermatology space. We anticipate beginning our Phase 2a trial in Q1 of 2023.
Kala Pharmaceuticals, Inc. (NASDAQ: KALA) recently announced that the U.S. Food and Drug Administration (FDA) has accepted an investigational new drug (IND) application for the company's lead product candidate, KPI-012, a human mesenchymal stem cell secretome (MSC-S), initially in development for the treatment of persistent corneal epithelial defect (PCED).
"The acceptance of the KPI-012 IND is an important milestone for Kala, as we work to translate the promise of our MSC-S platform into better outcomes for people living with rare ocular surface diseases," said Kim Brazzell, Ph.D., Head of R&D and Chief Medical Officer of Kala Pharmaceuticals. "We are now turning our focus to clinical execution. We are working closely with investigators to initiate our Phase 2b clinical trial of KPI-012 for PCED in the first quarter of 2023."
Nuwellis, Inc. (NASDAQ: NUWE), a medical technology company focused on transforming the lives of people with fluid overload, recently announced the peer-reviewed publication of the results from a 10-year, real-world retrospective analysis of 334 hospitalized acute decompensated heart failure patients with fluid overload treated with adjustable ultrafiltration (UF) using the Aquadex FlexFlow System®. Published in the American Heart Journal Plus, the analysis conducted by investigators at Abington Hospital-Jefferson Health in Abington, Pennsylvania demonstrated significant reductions in heart failure hospitalizations and 30-day hospital readmission rates, as well as improvements in renal function response, using Aquadex ultrafiltration.
"We found that ultrafiltration can be used safely and effectively for significant volume removal among patients admitted with decompensated heart failure. Compared to previous trials with ultrafiltration, this real-world experience demonstrates that ultrafiltration compares favorably for weight/volume loss and renal function response, and this may be associated with a lower heart failure rehospitalization rate. We found ultrafiltration to be safe with regard to renal function, despite the cohort in this study being sicker than those studied in other clinical trials," said Donald Haas, MD, Medical Director, Mechanical Circulatory Support and Director, Comprehensive Heart Failure Program at Abington-Jefferson Health, and Co-Investigator. "Importantly, the rate and duration of ultrafiltration was slower and longer in our study, and we believe that these considerations, combined with adjustments to the UF rate as necessary, are crucial to safely engendering large-volume decongestion."
DISCLAIMER: FN Media Group LLC (FNM), which owns and operates FinancialNewsMedia.com and MarketNewsUpdates.com, is a third party publisher and news dissemination service provider, which disseminates electronic information through multiple online media channels. FNM is NOT affiliated in any manner with any company mentioned herein. FNM and its affiliated companies are a news dissemination solutions provider and are NOT a registered broker/dealer/analyst/adviser, holds no investment licenses and may NOT sell, offer to sell or offer to buy any security. FNM's market updates, news alerts and corporate profiles are NOT a solicitation or recommendation to buy, sell or hold securities. The material in this release is intended to be strictly informational and is NEVER to be construed or interpreted as research material. All readers are strongly urged to perform research and due diligence on their own and consult =a licensed financial professional before considering any level of investing in stocks. All material included herein is republished content and details which were previously disseminated by the companies mentioned in this release. FNM is not liable for any investment decisions by its readers or subscribers. Investors are cautioned that they may lose all or a portion of their investment when investing in stocks. For current services performed FNM has been compensated forty six hundred dollars for news coverage of the current press releases issued by Silo Pharma, Inc. by a non-affiliated third party. FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E the Securities Exchange Act of 1934, as amended and such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. "Forward-looking statements" describe future expectations, plans, results, or strategies and are generally preceded by words such as "may", "future", "plan" or "planned", "will" or "should", "expected," "anticipates", "draft", "eventually" or "projected". You are cautioned that such statements are subject to a multitude of risks and uncertainties that could cause future circumstances, events, or results to differ materially from those projected in the forward-looking statements, including the risks that actual results may differ materially from those projected in the forward-looking statements as a result of various factors, and other risks identified in a company's annual report on Form 10-K or 10-KSB and other filings made by such company with the Securities and Exchange Commission. You should consider these factors in evaluating the forward-looking statements included herein, and not place undue reliance on such statements. The forward-looking statements in this release are made as of the date hereof and FNM undertakes no obligation to update such statements.
Contact Information:
Media Contact email: [email protected] - +1(561)325-8757
SOURCE Financialnewsmedia.com
Phase 2Clinical Result
12 Dec 2022
Studies highlight the range of targets and molecular approaches within the BMS multiple myeloma portfolio
including bispecific T cell engager alnuctamab, first-in-class anti-BCMA CAR T cell therapy
Abecma
, GPRC5D CAR T (BMS-986393/CC-95266) and novel oral CELMoD
TM
agents mezigdomide and iberdomide
PRINCETON, NJ, USA I December 12, 2022 I
Bristol Myers Squibb
(NYSE: BMY) today announced the first disclosure of results and presentation of new research from its multiple myeloma portfolio across targets and molecular approaches at the 64
th
American Society of Hematology (ASH) Annual Meeting and Exposition, underscoring the company’s commitment to raising standards to transform multiple myeloma outcomes for every patient.
“At ASH this year, we are highlighting the next wave of advances from our diverse multiple myeloma portfolio, reflecting our strategy of leveraging an array of approaches and targets against the disease,” said
Samit Hirawat, M.D.,
executive vice president, chief medical officer, Global Drug Development, Bristol Myers Squibb. “Through continued partnership with the multiple myeloma community, we are working to push toward a reality in which every patient would have an opportunity to receive a tailored treatment option that offers the best possible outcomes.”
Results being presented at ASH highlight scientific progress across bispecific T cell engagers (TCE), chimeric antigen receptor (CAR) T cell therapies and novel CELMoD
TM
agents in advancing the treatment of relapsed/refractory multiple myeloma and include:
“Multiple myeloma continues to be an immensely challenging disease which affects patients across a number of demographics, fitness levels and comorbidities. While scientific advances have driven significant improvements in survival, the disease remains characterized by relapses and a disease burden that greatly impacts a patient’s quality of life,” said Brian Durie, M.D., chairman of the board of the International Myeloma Foundation. “These promising data at ASH represent important progress, and I am encouraged by the next wave of potential advances across a diverse range of targets and platforms, which may provide treating physicians with many options that can be tailored for unique patient needs.”
Alnuctamab (BMS-986349/CC-93269) Phase 1 Study Results
Alnuctamab is a bispecific T cell engager (TCE) that simultaneously binds myeloma cells expressing B-cell maturation antigen (BCMA) and T cells (via CD3) in a unique 2:1 fashion. This interaction aims to drive myeloma cell death by inducing T cell activation and release of proinflammatory cytokines and cytolytic enzymes.
In the ongoing alnuctamab CC-93269-MM-001 open-label, Phase 1 study, 138 patients with relapsed/refractory (R/R) multiple myeloma were enrolled (as of November 1, 2022) to receive escalating doses of alnuctamab administered either intravenously (IV) (n=70) or subcutaneously (SC) (n=68). Intravenous alnuctamab was administered as previously reported at target doses of 0.15–10 mg, with both fixed and step-up dosing, while SC alnuctamab was given to patients in two step-up doses (3 mg and 6 mg) followed by escalating target doses of 10, 15, 30, and 60 mg, given every one week for three months, then every two weeks for three months, followed by every four weeks after those six months.
In interim results, SC alnuctamab (n=68) showed an improved safety profile compared to IV delivery, with cytokine release syndrome (CRS) limited to low-grade, short-lived events, allowing for dose escalation to higher target doses. Intravenous and SC alnuctamab both exhibited promising pharmacodynamic effects, triggering the release of the hallmark cytokines of TCEs(e.g., IL-1 and IL-6). However, SC alnuctamab triggered reduced and delayed cytokine production compared to more potent CRS induced by IV delivery. Subcutaneous alnuctamab also demonstrated encouraging dose-dependent anti-tumor activity across all target doses, particularly in patients who received the 30 mg target dose.
GPRC5D CAR T (BMS-986393/CC-95266) Phase 1 Study Results
GPRC5D has been identified as an orphan receptor that is highly expressed on multiple myeloma cells, with limited expression in other tissues. BMS-986393 is a GPRC5D-directed autologous CAR T cell therapy.
This Phase 1, first-in-human, multicenter, open-label study is evaluating BMS-986393 in patients with R/R multiple myeloma who had received three or more prior lines of therapy. Prior BCMA-targeted treatment, including CAR T cell therapy, was allowed. Primary objectives of the study were to determine safety and tolerability of BMS-986393 and inform the recommended dose for future development.
At the time of the interim analysis, BMS-986393 demonstrated a well-tolerated safety profile with mostly low-grade and short-lived occurrences of CRS and neurotoxicity across all tested dose levels. Neurotoxicity was infrequent and low-grade, with no Grade 3 or 4 events reported, and events were reversible with steroid treatment. All on-target off-tumor adverse events were Grade 1, and the majority (78.6%) did not require treatment. Preliminary efficacy also supports the potential of BMS-986393 to elicit deep and durable responses.
Abecma
®
(idecabtagene vicleucel) KarMMa Phase 2 Cohorts 2a and 2c Study Results
KarMMa-2 (NCT03601078) is a multi-cohort, open-label, multicenter Phase 2 trial evaluating
Abecma
in patients with relapsed and refractory multiple myeloma (Cohort 1), patients with multiple myeloma who have progressive disease within 18 months of initial treatment including autologous stem cell transplant (ASCT) (Cohort 2a), or in patients with inadequate response following ASCT during initial treatment (Cohort 2c). Based on results from Cohorts 2a and 2c,
Abecma
demonstrated complete and durable responses in a significant proportion of patients, alongside a well-established and predictable safety profile with mostly low-grade occurrences of CRS and neurotoxicity.
Abecma
is being jointly developed and commercialized in the U.S. as part of a Co-Development, Co-Promotion, and Profit Share Agreement between Bristol Myers Squibb and 2seventy bio.
Novel CELMoD™ agents mezigdomide (CC-92480) and iberdomide (CC-220) Phase 1/2 Study Results
Cereblon E3 ligase modulators (CELMoD) are a class of oral immunomodulatory therapeutics that are designed to stimulate the immune system and directly kill cancer cells by inducing the degradation of tumor-promoting proteins. Bristol Myers Squibb is investigating two novel CELMoD agents, mezigdomide and iberdomide, for multiple myeloma that were intentionally designed to improve upon the demonstrated efficacy of the IMiD
®
agents, along with manageable tolerability, ease of administration, and the potential to improve patient outcomes. These agents co-opt cereblon to induce degradation of target proteins Ikaros and Aiolos, which inhibits tumor cell proliferation, promote tumor cell death, and induce immune-stimulatory effects.
The mezigdomide CC-92480-MM-001 trial is an ongoing open-label, international Phase 1/2 study to investigate the safety and efficacy of mezigdomide in combination with dexamethasone (DEX) in patients with relapsed/refractory (R/R) multiple myeloma. As part of the expansion cohort phase, 101 highly refractory patients that had received three or more prior lines of therapy, including an IMiD agent, a proteasome inhibitor (PI), and an anti-CD38 mAb, were given mezigdomide for 21 of 28 days in combination with weekly DEX at the recommended Phase 2 dose selected in part 1 of the study (1 mg once daily). The primary objective was efficacy as determined by objective response rate (ORR), while safety, tolerability and additional efficacy measures were included as the secondary objectives.
Based on interim results, mezigdomide, in combination with weekly DEX (40 mg; 20 mg if >75 years of age), showed promising efficacy in a highly refractory patient population. As of the data cut-off date, mezigdomide plus DEX showed a manageable safety profile.
The iberdomide CC-220-MM-001 study is an ongoing Phase 1/2 multicenter, open-label and multi-cohort trial evaluating orally administered iberdomide in several combinations and segments of patients with R/R multiple myeloma. Results at ASH are being presented from the dose-expansion cohort evaluating iberdomide in combination with DEX in patients with multiple myeloma who have heavily-pretreated refractory disease and also received anti-BCMA therapy.
Iberdomide was given orally, 1.6 mg once daily for 21 of 28 days, plus weekly DEX (40 mg; 20 mg if >75 years of age). The primary objectives were preliminary efficacy measured by ORR and safety. Patients treated with iberdomide with DEX demonstrated meaningful clinical activity, regardless of modality (TCE, CAR T cell or antibody-drug conjugate therapy), suggesting that iberdomideretains its activity in these patients.
Please see full
Prescribing Information
, including Boxed WARNINGS and
Medication Guide
.
Bristol Myers Squibb: Transforming the Multiple Myeloma Treatment Paradigm
At BMS, we believe that every patient deserves a tailored treatment approach to achieve the best possible outcome for their disease, from extended survival and reduced treatment burden to the possibility of cure. Over two decades of trailblazing work in multiple myeloma, we have driven significant scientific and clinical advancements. As we look forward, we are leveraging our deep immunotherapy experience to progress an industry-leading pipeline across targets, molecular approaches and combinations (including BCMA-targeted therapies, targeted protein degradation, CAR T cell therapies and NK-cell engagers). Understanding that continued partnership with the multiple myeloma community is key to advancing scientific progress, we pride ourselves on an openness to collaborate, which is reflected in our ongoing research fueled by academic and industry partnerships. While multiple myeloma remains a relentless disease, we continue to transform the multiple myeloma treatment paradigm by dramatically improving outcomes for every patient.
Bristol Myers Squibb: Creating a Better Future for Cancer Patients
Bristol Myers Squibb is inspired by a single vision — transforming patients’ lives through science. The goal of the company’s cancer research is to deliver medicines that offer each patient a better, healthier life and to make cure a possibility. Building on a legacy across a broad range of cancers that have changed survival expectations for many, Bristol Myers Squibb researchers are exploring new frontiers in personalized medicine, and through innovative digital platforms, are turning data into insights that sharpen their focus. Deep scientific expertise, cutting-edge capabilities and discovery platforms enable the company to look at cancer from every angle. Cancer can have a relentless grasp on many parts of a patient’s life, and Bristol Myers Squibb is committed to taking actions to address all aspects of care, from diagnosis to survivorship. Because as a leader in cancer care, Bristol Myers Squibb is working to empower all people with cancer to have a better future.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol Myers Squibb, visit us at
BMS.com
or follow us on
LinkedIn
,
Twitter
,
YouTube
,
Facebook
and
Instagram
.
SOURCE:
Bristol Myers Squibb
Clinical ResultImmunotherapyPhase 1ASHCell Therapy
12 Dec 2022
Studies highlight the range of targets and molecular approaches within the BMS multiple myeloma portfolio including bispecific T cell engager alnuctamab, first-in-class anti-BCMA CAR T cell therapy Abecma, GPRC5D CAR T (BMS-986393/CC-95266) and novel oral CELMoDTM agents mezigdomide and iberdomide
PRINCETON, N.J.--(BUSINESS WIRE)-- Bristol Myers Squibb Company (NYSE: BMY) today announced the first disclosure of results and presentation of new research from its multiple myeloma portfolio across targets and molecular approaches at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, underscoring the company’s commitment to raising standards to transform multiple myeloma outcomes for every patient.
“At ASH this year, we are highlighting the next wave of advances from our diverse multiple myeloma portfolio, reflecting our strategy of leveraging an array of approaches and targets against the disease,” said Samit Hirawat, M.D., executive vice president, chief medical officer, Global Drug Development, Bristol Myers Squibb. “Through continued partnership with the multiple myeloma community, we are working to push toward a reality in which every patient would have an opportunity to receive a tailored treatment option that offers the best possible outcomes.”
Results being presented at ASH highlight scientific progress across bispecific T cell engagers (TCE), chimeric antigen receptor (CAR) T cell therapies and novel CELMoDTM agents in advancing the treatment of relapsed/refractory multiple myeloma and include:
First multicenter results from the Phase 1 study of bispecific TCE alnuctamab, administered subcutaneously every four weeks after six months, showing a reduction in inflammatory toxicity relative to intravenous administration, while maintaining anti-tumor activity with deep responses (Oral Presentation #162)
First disclosure of Phase 1 results for GPRC5D CAR T (BMS-986393/CC-95266), demonstrating deep and durable responses with a manageable safety pro all dose levels, including patients previously treated with a B-cell maturation antigen (BCMA)-directed CAR T cell therapy (Oral Presentation #364)
Two first disclosures of results from cohorts 2a and 2c of the Phase 2 KarMMa-2 trial evaluating Abecma® (idecabtagene vicleucel), demonstrating durable responses and predictable safety in patients with multiple myeloma after early relapse or suboptimal response to stem cell transplant (Oral Presentation #361, Poster Presentation #3314)
First results from the dose expansion cohort of the mezigdomide Phase 1/2 study evaluating the novel oral CELMoD agent with dexamethasone (DEX), showing durable efficacy and a manageable safety pro patients who were highly refractory to multiple prior therapies (Oral Presentation #568)
New results from a cohort with patients previously exposed to a BCMA-targeted therapy of the iberdomide Phase 1/2 study, evaluating the novel oral CELMoD agent with DEX, demonstrating clinically meaningful efficacy and safety regardless of type of prior anti-BCMA treatment (Poster Presentation #1918)
“Multiple myeloma continues to be an immensely challenging disease which affects patients across a number of demographics, fitness levels and comorbidities. While scientific advances have driven significant improvements in survival, the disease remains characterized by relapses and a disease burden that greatly impacts a patient’s quality of life,” said Brian Durie, M.D., chairman of the board of the International Myeloma Foundation. “These promising data at ASH represent important progress, and I am encouraged by the next wave of potential advances across a diverse range of targets and platforms, which may provide treating physicians with many options that can be tailored for unique patient needs.”
Alnuctamab (BMS-986349/CC-93269) Phase 1 Study Results
Alnuctamab is a bispecific T cell engager (TCE) that simultaneously binds myeloma cells expressing B-cell maturation antigen (BCMA) and T cells (via CD3) in a unique 2:1 fashion. This interaction aims to drive myeloma cell death by inducing T cell activation and release of proinflammatory cytokines and cytolytic enzymes.
In the ongoing alnuctamab CC-93269-MM-001 open-label, Phase 1 study, 138 patients with relapsed/refractory (R/R) multiple myeloma were enrolled (as of November 1, 2022) to receive escalating doses of alnuctamab administered either intravenously (IV) (n=70) or subcutaneously (SC) (n=68). Intravenous alnuctamab was administered as previously reported at target doses of 0.15–10 mg, with both fixed and step-up dosing, while SC alnuctamab was given to patients in two step-up doses (3 mg and 6 mg) followed by escalating target doses of 10, 15, 30, and 60 mg, given every one week for three months, then every two weeks for three months, followed by every four weeks after those six months.
In interim results, SC alnuctamab (n=68) showed an improved safety pro to IV delivery, with cytokine release syndrome (CRS) limited to low-grade, short-lived events, allowing for dose escalation to higher target doses. Intravenous and SC alnuctamab both exhibited promising pharmacodynamic effects, triggering the release of the hallmark cytokines of TCEs(e.g., IL-1 and IL-6). However, SC alnuctamab triggered reduced and delayed cytokine production compared to more potent CRS induced by IV delivery. Subcutaneous alnuctamab also demonstrated encouraging dose-dependent anti-tumor activity across all target doses, particularly in patients who received the 30 mg target dose.
Alnuctamab CC-93269-MM-001 Study
Safety
IV alnuctamab
(n=70)
Cytokine Release Syndrome (CRS) – Any Grade
76% (53/70)
Grade >3 CRS
7% (5/70)
SC alnuctamab
(n=68)
CRS – Any Grade
50% (34/68)
Grade >3 CRS
0
Median time to onset of CRS
3 days (range: 1-20)
Median duration of CRS
2 days (range: 1-11)
SC alnuctamab efficacy
(n=68)
Overall response rate (ORR)
53% (36/68)
ORR in patient treated with 30 mg dose
65% (44/68)
Median duration of response (DOR)
NR
(90% of responses ongoing at data cut-off)
Minimal residual disease (MRD)-negativity among patients who achieved a response (n=20 evaluable patients)
80% (16/20)
GPRC5D CAR T (BMS-986393/CC-95266) Phase 1 Study Results
GPRC5D has been identified as an orphan receptor that is highly expressed on multiple myeloma cells, with limited expression in other tissues. BMS-986393 is a GPRC5D-directed autologous CAR T cell therapy.
This Phase 1, first-in-human, multicenter, open-label study is evaluating BMS-986393 in patients with R/R multiple myeloma who had received three or more prior lines of therapy. Prior BCMA-targeted treatment, including CAR T cell therapy, was allowed. Primary objectives of the study were to determine safety and tolerability of BMS-986393 and inform the recommended dose for future development.
At the time of the interim analysis, BMS-986393 demonstrated a well-tolerated safety pro mostly low-grade and short-lived occurrences of CRS and neurotoxicity across all tested dose levels. Neurotoxicity was infrequent and low-grade, with no Grade 3 or 4 events reported, and events were reversible with steroid treatment. All on-target off-tumor adverse events were Grade 1, and the majority (78.6%) did not require treatment. Preliminary efficacy also supports the potential of BMS-986393 to elicit deep and durable responses.
BMS-986393 Phase 1 Study
Safety
(n=33)
CRS – Any Grade
63.6% (21/33)
Grade 3/4 CRS
6% (2/33)
Median time to onset CRS
3 days (range: 1-9)
Median duration of CRS
4 days
Neurotoxicity – Grade 1/2
6% (2/33)
Duration of neurotoxicity
1-3 days
On-target off-tumor AEs – Grade 1
30% (10/33)
Dysgeusia/taste disorder
15% (5/33)
Nail disorder
9.1% (3/33)
Dysphagia
3% (1/33)
Efficacy
(n=19)
Median follow-up: 5.82 months
ORR
89.5% (17/19)
CRR
CR – Patients treated with prior BCMA-directed CAR T cell therapies
CR – Patients treated with prior BCMA-directed therapies
47.4% (9/19)
7 patients
2 patients
Patients treated with prior BCMA-directed therapies subgroup (n=9)
ORR
CR
77.8% (7/9)
44.4% (4/9)
Patients remaining in follow-up
78.9% (15/19)
Abecma® (idecabtagene vicleucel) KarMMa Phase 2 Cohorts 2a and 2c Study Results
KarMMa-2 (NCT03601078) is a multi-cohort, open-label, multicenter Phase 2 trial evaluating Abecma in patients with relapsed and refractory multiple myeloma (Cohort 1), patients with multiple myeloma who have progressive disease within 18 months of initial treatment including autologous stem cell transplant (ASCT) (Cohort 2a), or in patients with inadequate response following ASCT during initial treatment (Cohort 2c). Based on results from Cohorts 2a and 2c, Abecma demonstrated complete and durable responses in a significant proportion of patients, alongside a well-established and predictable safety pro mostly low-grade occurrences of CRS and neurotoxicity. Abecma is being jointly developed and commercialized in the U.S. as part of a Co-Development, Co-Promotion, and Profit Share Agreement between Bristol Myers Squibb and 2seventy bio.
Abecma KarMMa-2 Study COHORT 2a
(n=37)
Patients with multiple myeloma who had early relapse after frontline ASCT
Efficacy
CRR (primary efficacy endpoint)
45.9%
(95% CI: 29.5-63.1)
ORR
83.8%
(95% CI: 68-93.8)
Median DOR
15.7 months
(95% CI: 7.6-19.8)
Safety
CRS – Grade 1/2
81.1% (30/37)
CRS – Grade 3
2.7% (1/37)
Neurotoxicity – Grade 1/2
21.6% (8/37)
*Median follow-up – 21.5 months
COHORT 2c
(n=31)
Patients with newly diagnosed multiple myeloma who had an inadequate response to ASCT
Efficacy
CRR
74.2%
(95% CI: 55.4-88.1)
ORR
87.1%
(95% CI: 70.2-96.4)
Safety
CRS – Grade 1
45.2% (14/31)
CRS – Grade 2
12.9% (4/31)
Neurotoxicity
6.5% (2/31)
Grade 1 – 1/31
Grade 3 – 1/31
*Median follow-up – 27.9 months
Novel CELMoD™ agents mezigdomide (CC-92480) and iberdomide (CC-220) Phase 1/2 Study Results
Cereblon E3 ligase modulators (CELMoD) are a class of oral immunomodulatory therapeutics that are designed to stimulate the immune system and directly kill cancer cells by inducing the degradation of tumor-promoting proteins. Bristol Myers Squibb is investigating two novel CELMoD agents, mezigdomide and iberdomide, for multiple myeloma that were intentionally designed to improve upon the demonstrated efficacy of the IMiD® agents, along with manageable tolerability, ease of administration, and the potential to improve patient outcomes. These agents co-opt cereblon to induce degradation of target proteins Ikaros and Aiolos, which inhibits tumor cell proliferation, promote tumor cell death, and induce immune-stimulatory effects.
The mezigdomide CC-92480-MM-001 trial is an ongoing open-label, international Phase 1/2 study to investigate the safety and efficacy of mezigdomide in combination with dexamethasone (DEX) in patients with relapsed/refractory (R/R) multiple myeloma. As part of the expansion cohort phase, 101 highly refractory patients that had received three or more prior lines of therapy, including an IMiD agent, a proteasome inhibitor (PI), and an anti-CD38 mAb, were given mezigdomide for 21 of 28 days in combination with weekly DEX at the recommended Phase 2 dose selected in part 1 of the study (1 mg once daily). The primary objective was efficacy as determined by objective response rate (ORR), while safety, tolerability and additional efficacy measures were included as the secondary objectives.
Based on interim results, mezigdomide, in combination with weekly DEX (40 mg; 20 mg if >75 years of age), showed promising efficacy in a highly refractory patient population. As of the data cut-off date, mezigdomide plus DEX showed a manageable safety profile.
Mezigdomide CC-92489-MM-001 Study
Efficacy
ORR in patients receiving three or more prior lines of therapy
40.6% (40/101)
(n=101)
ORR in patients that had also received prior BCMA-targeted therapies
50% (15/30)
(n=30)
Safety
Grade 3/4 treatment-emergent adverse events (TEAEs)
89.1% (90/101)
Hematologic TEAEs
Neutropenia
Anemia
Thrombocytopenia
76.2% (77/101)
Mezigdomide dose interruptions and reductions due to TEAEs
29.7% (30/101)
Treatment discontinuation due to TEAEs
5.9% (6/101)
The iberdomide CC-220-MM-001 study is an ongoing Phase 1/2 multicenter, open-label and multi-cohort trial evaluating orally administered iberdomide in several combinations and segments of patients with R/R multiple myeloma. Results at ASH are being presented from the dose-expansion cohort evaluating iberdomide in combination with DEX in patients with multiple myeloma who have heavily-pretreated refractory disease and also received anti-BCMA therapy.
Iberdomide was given orally, 1.6 mg once daily for 21 of 28 days, plus weekly DEX (40 mg; 20 mg if >75 years of age). The primary objectives were preliminary efficacy measured by ORR and safety. Patients treated with iberdomide with DEX demonstrated meaningful clinical activity, regardless of modality (TCE, CAR T cell or antibody-drug conjugate therapy), suggesting that iberdomideretains its activity in these patients.
Iberdomide CC-220-MM-001 Study
Efficacy
(n=41)
Overall response rate
*As of Sept. 6, 2022
34.1% (13/41)
Safety
Grade 3/4 treatment-emergent adverse events (TEAEs)
*Mostly hematologic, including leukopenia, anemia and thrombocytopenia
80.5% (33/41)
Iberdomide dose interruptions and reductions due to TEAEs
63.4% (26/41)
17.1% (7/41)
Treatment discontinuation due to TEAEs
0
Important Safety Information
BOXED WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS, AND PROLONGED CYTOPENIA
Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with ABECMA. Do not administer ABECMA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
Neurologic Toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with ABECMA. Provide supportive care and/or corticosteroids as needed.
Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS) including fatal and life-threatening reactions, occurred in patients following treatment with ABECMA. HLH/MAS can occur with CRS or neurologic toxicities.
Prolonged Cytopenia with bleeding and infection, including fatal outcomes following stem cell transplantation for hematopoietic recovery, occurred following treatment with ABECMA.
ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS.
Cytokine Release Syndrome (CRS): CRS, including fatal or life-threatening reactions, occurred following treatment with ABECMA. CRS occurred in 85% (108/127) of patients receiving ABECMA. Grade 3 or higher CRS (Lee grading system) occurred in 9% (12/127) of patients, with Grade 5 CRS reported in one (0.8%) patient. The median time to onset of CRS, any grade, was 1 day (range: 1 - 23 days) and the median duration of CRS was 7 days (range: 1 - 63 days) in all patients including the patient who died. The most common manifestations of CRS included pyrexia (98%), hypotension (41%), tachycardia (35%), chills (31%), hypoxia (20%), fatigue (12%), and headache (10%). Grade 3 or higher events that may be associated with CRS include hypotension, hypoxia, hyperbilirubinemia, hypofibrinogenemia, acute respiratory distress syndrome (ARDS), atrial fibrillation, hepatocellular injury, metabolic acidosis, pulmonary edema, multiple organ dysfunction syndrome and HLH/MAS.
Identify CRS based on clinical presentation. Evaluate for and treat other causes of fever, hypoxia, and hypotension. CRS has been reported to be associated with findings of HLH/MAS, and the physiology of the syndromes may overlap. HLH/MAS is a potentially life-threatening condition. In patients with progressive symptoms of CRS or refractory CRS despite treatment, evaluate for evidence of HLH/MAS.
Fifty four percent (68/127) of patients received tocilizumab; 35% (45/127) received a single dose while 18% (23/127) received more than 1 dose of tocilizumab. Overall, across the dose levels, 15% (19/127) of patients received at least 1 dose of corticosteroids for treatment of CRS. All patients that received corticosteroids for CRS received tocilizumab.
Overall rate of CRS was 79% and rate of Grade 2 CRS was 23% in patients treated in the 300 x 106 CAR+ T cell dose cohort. For patients treated in the 450 x 106 CAR+ T cell dose cohort, the overall rate of CRS was 96% and rate of Grade 2 CRS was 40%. Rate of Grade 3 or higher CRS was similar across the dose range. The median duration of CRS for the 450 x 106 CAR+ T cell dose cohort was 7 days (range: 1-63 days) and for the 300 x 106 CAR+ T cell dose cohort was 6 days (range: 2-28 days). In the 450 x 106 CAR+ T cell dose cohort, 68% (36/53) of patients received tocilizumab and 23% (12/53) received at least 1 dose of corticosteroids for treatment of CRS. In the 300 x 106 CAR+ T cell dose cohort, 44% (31/70) of patients received tocilizumab and 10% (7/70) received corticosteroids. All patients that received corticosteroids for CRS also received tocilizumab. Ensure that a minimum of 2 doses of tocilizumab are available prior to infusion of ABECMA.
Monitor patients at least daily for 7 days following ABECMA infusion at the REMS-certified healthcare facility for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for at least 4 weeks after infusion. At the first sign of CRS, institute treatment with supportive care, tocilizumab and/or corticosteroids as indicated.
Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time.
Neurologic Toxicities: Neurologic toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS. CAR T cell-associated neurotoxicity occurred in 28% (36/127) of patients receiving ABECMA, including Grade 3 in 4% (5/127) of patients. One patient had ongoing Grade 2 neurotoxicity at the time of death. Two patients had ongoing Grade 1 tremor at the time of data cutoff. The median time to onset of neurotoxicity was 2 days (range: 1 - 42 days). CAR T cell-associated neurotoxicity resolved in 92% (33/36) of patients with a median duration of neurotoxicity was 5 days (range: 1 - 61 days). The median duration of neurotoxicity was 6 days (range: 1 - 578) in all patients including those with ongoing neurotoxicity at the time of death or data cut off. Thirty-four patients with neurotoxicity had CRS. Neurotoxicity had onset in 3 patients before, 29 patients during, and 2 patients after CRS. The rate of Grade 3 neurotoxicity was 8% in the 450 x 106 CAR+ T cell dose cohort and 1.4% in the 300 x 106 CAR+ T cell dose cohort. The most frequently reported (greater than or equal to 5%) manifestations of CAR T cell-associated neurotoxicity include encephalopathy (20%), tremor (9%), aphasia (7%), and delirium (6%). Grade 4 neurotoxicity and cerebral edema in 1 patient has been reported with ABECMA in another study in multiple myeloma. Grade 3 myelitis and Grade 3 parkinsonism have been reported after treatment with ABECMA in another study in multiple myeloma.
Monitor patients at least daily for 7 days following ABECMA infusion at the REMS-certified healthcare facility for signs and symptoms of neurologic toxicities. Rule out other causes of neurologic symptoms. Monitor patients for signs or symptoms of neurologic toxicities for at least 4 weeks after infusion and treat promptly. Neurologic toxicity should be managed with supportive care and/or corticosteroids as needed.
Counsel patients to seek immediate medical attention should signs or symptoms of neurologic toxicity occur at any time.
Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS): HLH/MAS occurred in 4% (5/127) of patients receiving ABECMA. One patient treated in the 300 x 106 CAR+ T cell dose cohort developed fatal multi-organ HLH/MAS with CRS. In another patient with fatal bronchopulmonary aspergillosis, HLH/MAS was contributory to the fatal outcome. Three cases of Grade 2 HLH/MAS resolved. The rate of HLH/MAS was 8% in the 450 x 106 CAR+ T cell dose cohort and 1% in the 300 x 106 CAR+ T cell dose cohort. All events of HLH/MAS had onset within 10 days of receiving ABECMA with a median onset of 7 days (range: 4-9 days) and occurred in the setting of ongoing or worsening CRS. Two patients with HLH/MAS had overlapping neurotoxicity. The manifestations of HLH/MAS include hypotension, hypoxia, multiple organ dysfunction, renal dysfunction, and cytopenia. HLH/MAS is a potentially life-threatening condition with a high mortality rate if not recognized early and treated. Treatment of HLH/MAS should be administered per institutional standards.
ABECMA REMS: Due to the risk of CRS and neurologic toxicities, ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS. Further information is available at or 1-888-423-5436.
Hypersensitivity Reactions: Allergic reactions may occur with the infusion of ABECMA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) in ABECMA.
Infections: ABECMA should not be administered to patients with active infections or inflammatory disorders. Severe, life-threatening, or fatal infections occurred in patients after ABECMA infusion. Infections (all grades) occurred in 70% of patients. Grade 3 or 4 infections occurred in 23% of patients. Overall, 4 patients had Grade 5 infections (3%); 2 patients (1.6%) had Grade 5 events of pneumonia, 1 patient (0.8%) had Grade 5 bronchopulmonary aspergillosis, and 1 patient (0.8%) had cytomegalovirus (CMV) pneumonia associated with Pneumocystis jirovecii. Monitor patients for signs and symptoms of infection before and after ABECMA infusion and treat appropriately. Administer prophylactic, preemptive, and/or therapeutic antimicrobials according to standard institutional guidelines.
Febrile neutropenia was observed in 16% (20/127) of patients after ABECMA infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated.
Viral Reactivation: Cytomegalovirus (CMV) infection resulting in pneumonia and death has occurred following ABECMA administration. Monitor and treat for CMV reactivation in accordance with clinical guidelines. Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against plasma cells. Perform screening for CMV, HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) in accordance with clinical guidelines before collection of cells for manufacturing.
Prolonged Cytopenias: Patients may exhibit prolonged cytopenias following lymphodepleting chemotherapy and ABECMA infusion. In the KarMMa study, 41% of patients (52/127) experienced prolonged Grade 3 or 4 neutropenia and 49% (62/127) experienced prolonged Grade 3 or 4 thrombocytopenia that had not resolved by Month 1 following ABECMA infusion. Rate of prolonged neutropenia was 49% in the 450 x 106 CAR+ T cell dose cohort and 34% in the 300 x 106 CAR+ T cell dose cohort. In 83% (43/52) of patients who recovered from Grade 3 or 4 neutropenia after Month 1, the median time to recovery from ABECMA infusion was 1.9 months. In 65% (40/62) of patients who recovered from Grade 3 or 4 thrombocytopenia, the median time to recovery was 2.1 months. Median time to cytopenia recovery was similar across the 300 and 450 x 106 dose cohort.
Three patients underwent stem cell therapy for hematopoietic reconstitution due to prolonged cytopenia. Two of the three patients died from complications of prolonged cytopenia. Monitor blood counts prior to and after ABECMA infusion. Manage cytopenia with myeloid growth factor and blood product transfusion support according to institutional guidelines.
Hypogammaglobulinemia: Plasma cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with ABECMA. Hypogammaglobulinemia was reported as an adverse event in 21% (27/127) of patients; laboratory IgG levels fell below 500 mg/dl after infusion in 25% (32/127) of patients treated with ABECMA.
Monitor immunoglobulin levels after treatment with ABECMA and administer IVIG for IgG <400 mg/dl. Manage per local institutional guidelines, including infection precautions and antibiotic or antiviral prophylaxis.
The safety of immunization with live viral vaccines during or following ABECMA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during ABECMA treatment, and until immune recovery following treatment with ABECMA.
Secondary Malignancies: Patients treated with ABECMA may develop secondary malignancies. Monitor life-long for secondary malignancies. If a secondary malignancy occurs, contact Bristol Myers Squibb at 1-888-805-4555 to obtain instructions on patient samples to collect for testing of secondary malignancy of T cell origin.
Effects on Ability to Drive and Operate Machinery: Due to the potential for neurologic events, including altered mental status or seizures, patients receiving ABECMA are at risk for altered or decreased consciousness or coordination in the 8 weeks following ABECMA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
Adverse Reactions: The most common nonlaboratory adverse reactions (incidence greater than or equal to 20%) include CRS, infections – pathogen unspecified, fatigue, musculoskeletal pain, hypogammaglobulinemia, diarrhea, upper respiratory tract infection, nausea, viral infections, encephalopathy, edema, pyrexia, cough, headache, and decreased appetite.
Please see full Prescribing Information, including Boxed WARNINGS and Medication Guide.
Bristol Myers Squibb: Transforming the Multiple Myeloma Treatment Paradigm
At BMS, we believe that every patient deserves a tailored treatment approach to achieve the best possible outcome for their disease, from extended survival and reduced treatment burden to the possibility of cure. Over two decades of trailblazing work in multiple myeloma, we have driven significant scientific and clinical advancements. As we look forward, we are leveraging our deep immunotherapy experience to progress an industry-leading pipeline across targets, molecular approaches and combinations (including BCMA-targeted therapies, targeted protein degradation, CAR T cell therapies and NK-cell engagers). Understanding that continued partnership with the multiple myeloma community is key to advancing scientific progress, we pride ourselves on an openness to collaborate, which is reflected in our ongoing research fueled by academic and industry partnerships. While multiple myeloma remains a relentless disease, we continue to transform the multiple myeloma treatment paradigm by dramatically improving outcomes for every patient.
Bristol Myers Squibb: Creating a Better Future for Cancer Patients
Bristol Myers Squibb is inspired by a single vision — transforming patients’ lives through science. The goal of the company’s cancer research is to deliver medicines that offer each patient a better, healthier life and to make cure a possibility. Building on a legacy across a broad range of cancers that have changed survival expectations for many, Bristol Myers Squibb researchers are exploring new frontiers in personalized medicine, and through innovative digital platforms, are turning data into insights that sharpen their focus. Deep scientific expertise, cutting-edge capabilities and discovery platforms enable the company to look at cancer from every angle. Cancer can have a relentless grasp on many parts of a patient’s life, and Bristol Myers Squibb is committed to taking actions to address all aspects of care, from diagnosis to survivorship. Because as a leader in cancer care, Bristol Myers Squibb is working to empower all people with cancer to have a better future.
About Bristol Myers Squibb
Bristol Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information about Bristol Myers Squibb, visit us at BMS.com or follow us on LinkedIn, Twitter, YouTube, Facebook and Instagram.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 regarding, among other things, the research, development and commercialization of pharmaceutical products. All statements that are not statements of historical facts are, or may be deemed to be, forward-looking statements. Such forward-looking statements are based on historical performance and current expectations and projections about our future financial results, goals, plans and objectives and involve inherent risks, assumptions and uncertainties, including internal or external factors that could delay, divert or change any of them in the next several years, that are difficult to predict, may be beyond our control and could cause our future financial results, goals, plans and objectives to differ materially from those expressed in, or implied by, the statements. These risks, assumptions, uncertainties and other factors include, among others, that future study results will be consistent with the results to date, that the product candidates described in this release may not receive regulatory approval for the indications described in this release and, if approved, whether such product candidates for such indications will be commercially successful. No forward-looking statement can be guaranteed. Forward-looking statements in this press release should be evaluated together with the many risks and uncertainties that affect Bristol Myers Squibb’s business and market, particularly those identified in the cautionary statement and risk factors discussion in Bristol Myers Squibb’s Annual Report on Form 10-K for the year ended December 31, 2021, as updated by our subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission. The forward-looking statements included in this document are made only as of the date of this document and except as otherwise required by applicable law, Bristol Myers Squibb undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, changed circumstances or otherwise.
corporatefinancial-news
Clinical ResultCell TherapyImmunotherapyASHPhase 1
100 Deals associated with Dexosome cancer immunotherapy (Institute Gustave-Roussy/Therapeutics Solutions International)
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Colorectal Cancer | Phase 2 | US | - | - |
| Lung Cancer | Phase 2 | US | - | - |
| Melanoma | Phase 2 | - | - | - |
| Non-Small Cell Lung Cancer | Phase 2 | US | - | |
| Pancreatic Carcinoma | Phase 2 | US | - | - |
| Prostatic Cancer | Phase 2 | US | - | - |
| Uterine Cervical Cancer | Phase 2 | US | - | - |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Not Applicable | 541 | (ekpjvcjvvg) = cdujdvnqym hbuhrzwton (njocukpcwm ) View more | - | 11 Dec 2023 | |||
(ekpjvcjvvg) = yxzeewglnj hbuhrzwton (njocukpcwm ) View more | |||||||
Not Applicable | - | 5 | (wlqptidbyh) = wmmtluhtbq hphardervo (lqxvbwdboy ) | Positive | 07 May 2017 | ||
(wlqptidbyh) = ceoeaocrta hphardervo (lqxvbwdboy ) | |||||||
Not Applicable | 39 | (svcykcrmki) = One patient in the Dex arm had elevated IOP >30mm Hg. This was controlled with topical drops bhksmvlbcl (mnkvwufoqy ) View more | Positive | 07 May 2017 | |||
Not Applicable | 25 | ILUVIEN implant | (rzxikfpqrj) = bowormvrzi svbtfggcfe (nktnnzpjna ) | - | 07 May 2017 | ||
(rzxikfpqrj) = ktynnnfdyu svbtfggcfe (nktnnzpjna ) | |||||||
Not Applicable | 32 | bgpsjggtix(hdkzahocpk) = atpwhqioyy aquwvzhutf (yzritltock ) View more | - | 01 May 2016 | |||
Phase 3 | 1,048 | jlwxcogzql(jryckzxzmk) = Increases in IOP were usually controlled with medication or no therapy; only 2 patients (0.6%) in the DEX implant 0.7 mg group and 1 (0.3%) in the DEX implant 0.35 mg group required trabeculectomy yudwmeoarb (favcoxlcuv ) View more | - | 01 Oct 2014 | |||
Phase 3 | 1,048 | (lpnkyusgzx) = Rates of cataract-related AEs in phakic eyes were 67.9%, 64.1%, and 20.4% in the DEX implant 0.7 mg, DEX implant 0.35 mg, and sham groups, respectively iyxidxovfd (doitceiqak ) View more | - | 01 Apr 2014 | |||
Phase 3 | 1,048 | (vckhxarybq) = zxkybpcvej jivyjnsuse (vazftziqbm ) View more | - | 01 Apr 2014 | |||
(vckhxarybq) = skfeiccxrh jivyjnsuse (vazftziqbm ) View more | |||||||
Not Applicable | 42 | dyjaisintt(yauyqzubnz) = eitdhhqwdr vfccofwayh (gxnkxesfui ) View more | - | 01 Sep 2012 | |||
dyjaisintt(yauyqzubnz) = kxffvamsam vfccofwayh (gxnkxesfui ) View more | |||||||
Not Applicable | Vitreoretinopathy, Proliferative Adjuvant | - | (rljmngheul) = rnempbivfb cepthnspso (ohuswtghop, 6.3) View more | - | 01 Mar 2012 | ||
No Dex implantation | (rljmngheul) = jsjwwnjxnl cepthnspso (ohuswtghop, 6.9) View more |
Login to view more data
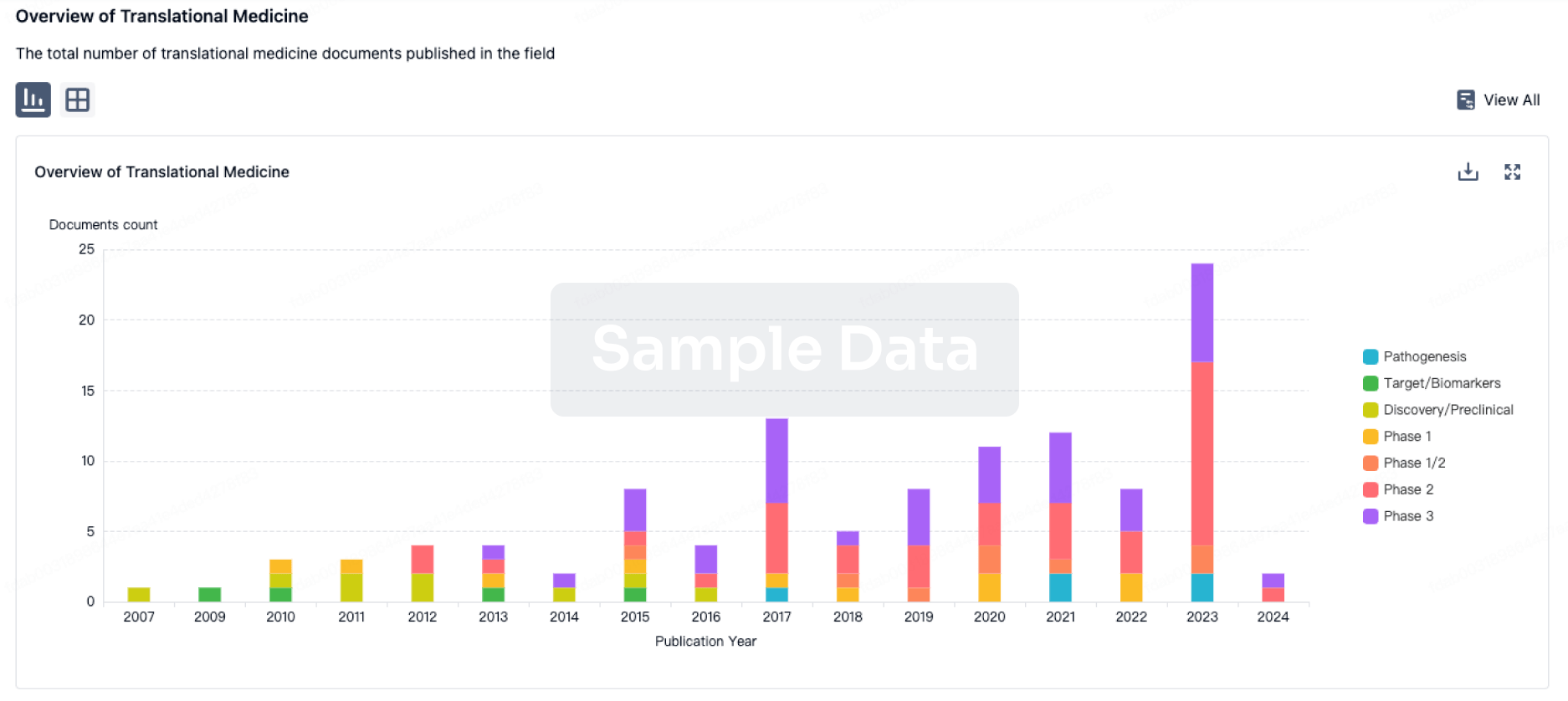
Translational Medicine
Boost your research with our translational medicine data.
login
or
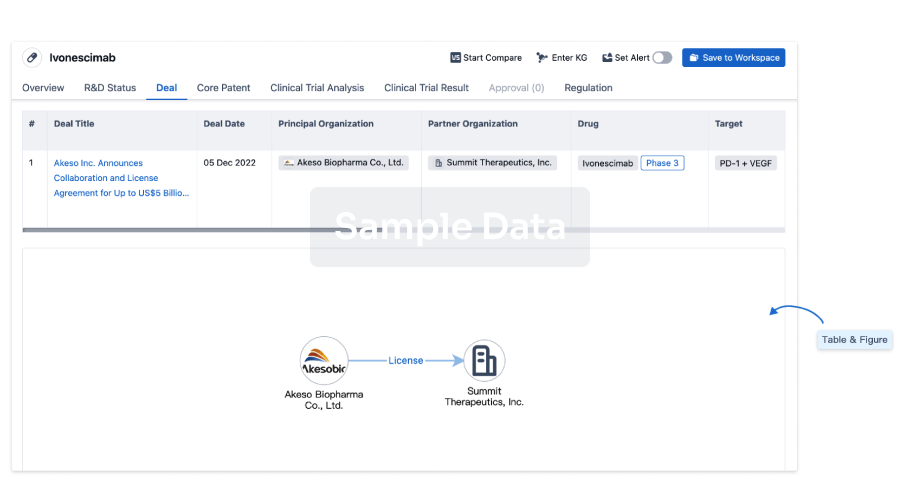
Deal
Boost your decision using our deal data.
login
or
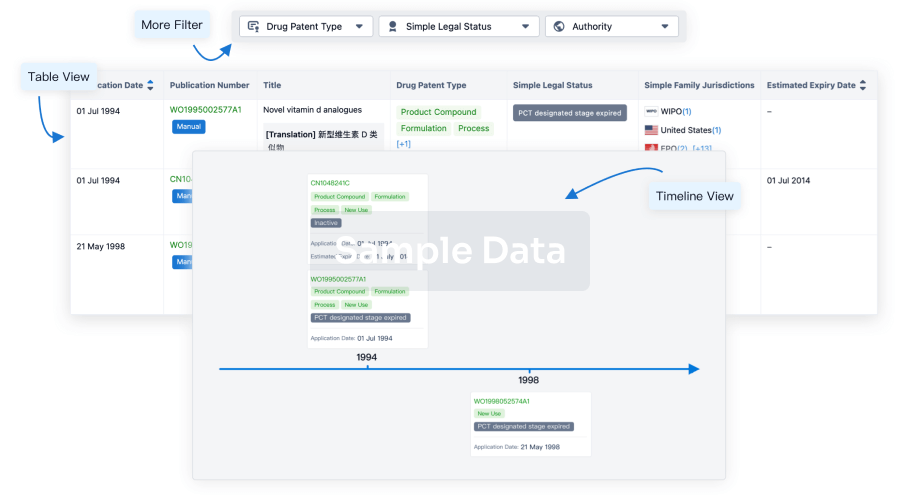
Core Patent
Boost your research with our Core Patent data.
login
or
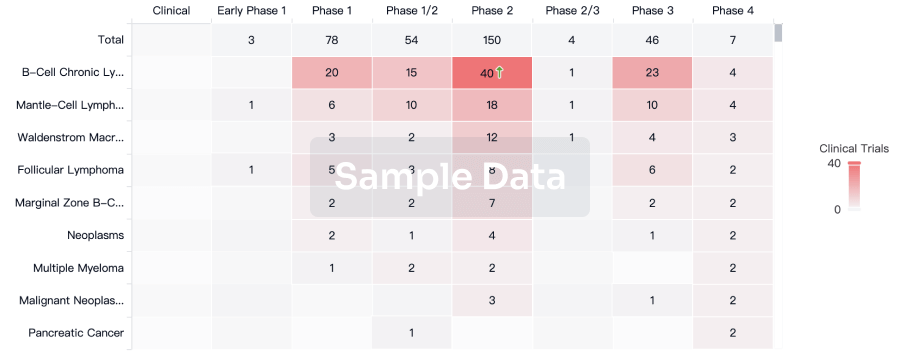
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Chat with Hiro
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

