SAN FRANCISCO and SUZHOU, China, March 30, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune, ophthalmology and other major diseases, announced that the Center for Drug Evaluation (CDE) of China's National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) for IBI363, a first-in-class PD-1/IL-2α-bias bispecific antibody fusion protein, as monotherapy for the treatment of unresectable locally advanced or metastatic mucosal or acral melanoma who have not received prior systemic therapy.
Recently, Innovent initiated and dosed the first patient for IBI363 in its first pivotal study to evaluate the efficacy and safety of IBI363 monotherapy versus pembrolizumab (Keytruda®) monotherapy in patients with unresectable, locally advanced or metastatic mucosal or acral melanoma who have not received prior systemic therapy. Furthermore, IBI363 has received two fast track designations (FTD) from the U.S. Food and Drug Administration (FDA), for the treatment of squamous non-small cell lung cancer and melanoma, respectively.
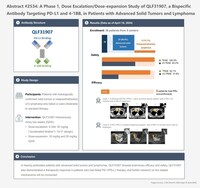
IBI363 has demonstrated outstanding efficacy signals in immunotherapy (IO)-naïve melanoma patients across two earlier clinical trials (Phase 1a/1b study NCT05460767 and Phase 2 study NCT06081920), which enrolled a total of 26 patients with advanced acral or mucosal melanoma:
The overall objective response rate (ORR) was 61.5%, and the disease control rate (DCR) was 84.6%—significantly higher than current domestic immunotherapy standards.
Prolonged follow-up revealed sustained tumor responses and long-term benefits, suggesting the potential superiority of IBI363 over existing standard therapies.
Dr. Hui Zhou, Senior Vice President of Innovent, said, "As Innovent's first-in-class next-generation IO therapy, IBI363 simultaneously and selectively inhibits the PD-1/PD-L1 pathway and activates the IL-2 pathway.
IBI363 has recently received multiple FTD and BTD designations, signifying regulatory recognition of its clinical value in addressing unmet medical needs. Non-cutaneous melanoma subtypes like mucosal melanoma—which are more prevalent in China—are particularly resistant to immunotherapy with limited clinical benefits[i]. We aim to validate IBI363's potential in its first pivotal trial, through a head-to-head comparison with pembrolizumab, as a superior treatment option for melanoma patients over the current standard-of-care. We are also accelerating the global development of IBI363 across multiple tumor types, with the goal of extending the benefits of China's innovation to patients worldwide."
NMPA Breakthrough Therapy Designation is intended to facilitate and expedite the development and review of investigational drugs for serious diseases or conditions when preliminary clinical evidence indicates substantial improvement over current therapies. BTD qualifies a drug candidate for accelerated review by the CDE and provides the sponsor with timely advice and communication to expedite the approval process, helping to address the unmet clinical needs of patients more swiftly.
About IBI363 (First-in-class PD-1/IL-2α-bias bispecific antibody fusion protein)
IBI363 is a first-in-class drug candidate independently developed by Innovent Biologics. It is a PD-1/IL-2 bispecific antibody fusion protein designed to enhance efficiency while minimizing toxicity. The IL-2 arm of IBI363 has been engineered to optimize therapeutic effects with reduced side effects, while the PD-1 binding arm enables PD-1 blockade and selective IL-2 delivery. By simultaneously inhibiting the PD-1/PD-L1 pathway and activating the IL-2 pathway, IBI363 facilitates more precise and efficient targeting and activation of tumor specific T cells. Preclinical studies have shown that IBI363 exhibits strong anti-tumor activity across multiple tumor-bearing pharmacological models, including those resistant to PD-1 inhibitors and metastatic models. Additionally, it has demonstrated a favorable safety profile in preclinical models.
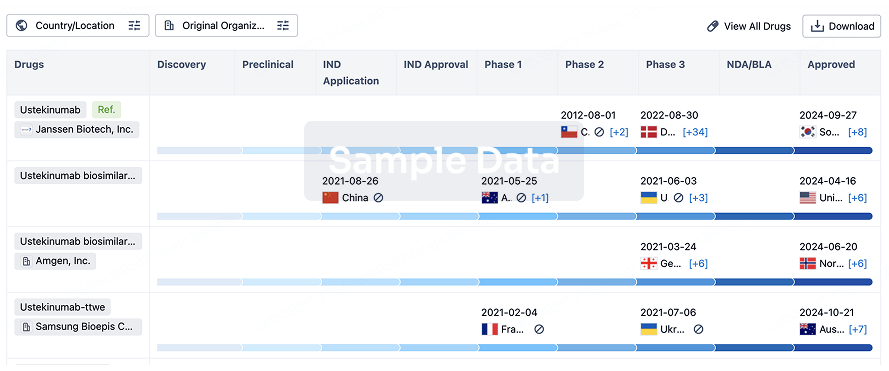
Clinical trials of IBI363 are currently underway in China, the United States, and Australia to evaluate its safety, tolerability and preliminary efficacy in subjects with advanced malignancies. The first pivotal study of IBI363 has been initiated, for the treatment of IO-naive mucosal or acral melanoma.
Furthermore, IBI363 has received two fast track designations (FTD) from the U.S. FDA, for the treatment of melanoma and squamous NSCLC, respectively. IBI363 has also received breakthrough therapy designation from the NMPA of China for the treatment of melanoma.
About Melanoma
Melanoma is a malignant tumor that develops from melanocytes. Although melanoma accounts for only 3% of all types of skin cancer, it has the highest mortality rate of all types and is the most likely to metastasize. In China, the incidence and mortality rate of melanoma continue to rise. Melanoma is classified into three main subtypes: cutaneous, acral and mucosal. The characteristics of melanoma in Chinese patients differs greatly from those seen in European and American Caucasian populations in terms of pathogenesis, biological behavior, histological morphology, treatment response and prognosis[i]. For advanced cutaneous and acral melanomas, patients with the BRAF V600 mutation typically receive BRAF inhibitor combined with MEK inhibitors as the preferred molecular targeted therapy. For those without the BRAF V600 mutation, chemotherapy combined with anti-angiogenic drugs can be is a first-line treatment option. Notably, pembrolizumab has been approved as the first-line treatment indication for advanced melanoma in September 2024 in China, although clinical benefits are limited. For second-line treatment, therapies not previously used in first-line settings are recommended. Patients who have not received PD-1 monoclonal antibody in the first-line setting may be treated with PD-1 inhibitors as a second-line option. For advanced mucosal melanoma -which are more prevalent in China-are particularly resistant to immunotherapy with limited clinical benefits, in urgent need of new treatment options.
About Innovent
Innovent is a leading biopharmaceutical company founded in 2011 with the mission to empower patients worldwide with affordable, high-quality biopharmaceuticals. The company discovers, develops, manufactures and commercializes innovative medicines that target some of the most intractable diseases. Its pioneering therapies treat cancer, cardiovascular and metabolic, autoimmune and eye diseases. Innovent has launched 15 products in the market. It has 3 new drug applications under regulatory review, 4 assets in Phase III or pivotal clinical trials and 15 more molecules in early clinical stage. Innovent partners with over 30 global healthcare companies, including Eli Lilly, Sanofi, Incyte, Adimab, LG Chem and MD Anderson Cancer Center.
Guided by the motto, "Start with Integrity, Succeed through Action," Innovent maintains the highest standard of industry practices and works collaboratively to advance the biopharmaceutical industry so that first-rate pharmaceutical drugs can become widely accessible. For more information, visit , or follow Innovent on Facebook and LinkedIn.
Statement:
(1)Innovent does not recommend the use of any unapproved drug (s)/indication (s).
(2)Ramucirumab (Cyramza), Selpercatinib (Retsevmo) and Pirtobrutinib (Jaypirca) were developed by Eli Lilly and Company.
Forward-Looking Statements
This news release may contain certain forward-looking statements that are, by their nature, subject to significant risks and uncertainties. The words "anticipate", "believe", "estimate", "expect", "intend" and similar expressions, as they relate to Innovent, are intended to identify certain of such forward-looking statements. Innovent does not intend to update these forward-looking statements regularly.
These forward-looking statements are based on the existing beliefs, assumptions, expectations, estimates, projections and understandings of the management of Innovent with respect to future events at the time these statements are made. These statements are not a guarantee of future developments and are subject to risks, uncertainties and other factors, some of which are beyond Innovent's control and are difficult to predict. Consequently, actual results may differ materially from information contained in the forward-looking statements as a result of future changes or developments in our business, Innovent's competitive environment and political, economic, legal and social conditions.
Innovent, the Directors and the employees of Innovent assume (a) no obligation to correct or update the forward-looking statements contained in this site; and (b) no liability in the event that any of the forward-looking statements does not materialize or turn out to be incorrect.
References:
[i] CSCO黑色素瘤诊疗指南(2023年版)
SOURCE Innovent Biologics
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED