Last update 16 May 2024
Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)
Last update 16 May 2024
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms Dexamethasone + [2] |
Target |
Mechanism GR agonists(Glucocorticoid receptor agonists) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
Drug Highest PhasePhase 2 |
First Approval Date- |
RegulationOrphan Drug (US) |
Structure
Molecular FormulaC22H29FO5 |
InChIKeyUREBDLICKHMUKA-CXSFZGCWSA-N |
CAS Registry50-02-2 |
Related
32
Clinical Trials associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)The efficacy of anti-VEGF combined with dexamethasone implant in the treatment of refractory diabetic macular edema
Start Date01 Jul 2023 |
Sponsor / Collaborator- |
The effectiveness of vitrecomy co-adjuvant intravitreal dexamethasone implant for proliferative diabetic retinopathy
Start Date25 Jun 2023 |
Sponsor / Collaborator- |
Ozurdex in Suboptimal Diabetic Macular Edema
The primary focus of this study is to understand the anatomic and visual outcomes of patients with refractory and suboptimal treatment response diabetic macular edema (DME) using anti-vascular endothelial growth factor (VEGF) to Ozurdex, an intravitreal dexamethasone implant. Secondly, investigators aim to understand the differences in cytokine profiles in patients who respond differently to intravitreal anti-VEGF versus Ozurdex. The importance of this study is to identify biomarkers that may help predict patients' response to different treatment protocols. Currently, Ozurdex is not covered by provincial health benefit plans for patients with DME. Our results may help improve access to care for those who have suboptimal results with or refractory to intravitreal anti-VEGF treatment.
Start Date25 May 2021 |
Sponsor / Collaborator- |
100 Clinical Results associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)
Login to view more data
100 Translational Medicine associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)
Login to view more data
100 Patents (Medical) associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)
Login to view more data
10
Literatures (Medical) associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)01 Jan 2023·Retina
PHASE 2 RANDOMIZED STUDY (ORION-1) OF A NOVEL, BIODEGRADABLE DEXAMETHASONE IMPLANT (AR-1105) FOR THE TREATMENT OF MACULAR EDEMA DUE TO CENTRAL OR BRANCH RETINAL VEIN OCCLUSION
Article
Author: Boyer, David S ; Williams, Stuart ; Hollander, David A ; Singer, Michael A ; Kopczynski, Casey C ; Trevino, Leo ; Kerr, Kevin ; Pegoraro, Tyler ; McKee, Hayley
01 Sep 2021·RetinaQ2 · MEDICINE
Correspondence
Q2 · MEDICINE
Letter
Author: Capasso, Luigi ; Gioia, Marco ; De Bernardo, Maddalena ; Rosa, Nicola
01 Sep 2021·RetinaQ2 · MEDICINE
Correspondence
Q2 · MEDICINE
Letter
Author: Saxena, Rushil Kumar ; Agarwal, Manisha ; Muralidhar, Alankrita
5
News (Medical) associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)12 Jun 2023
June 13, 2023 -- Repeat treatment with corticosteroid injections improved vision in people with persistent or recurrent uveitis-related macular edema better than two other therapies, according to results from a clinical trial funded by the National Eye Institute (NEI). Compared with methotrexate or ranibizumab intravitreal (in-the-eye) injections, the corticosteroid treatment achieved greater reductions in retinal swelling and was the only therapy in the study that improved vision. The report was published today in the journal
Ophthalmology
. NEI is part of the National Institutes of Health.
“Prior to this study, we didn’t know the best treatment for persistent or recurrent macular edema, a major cause of vision loss in people with uveitis,” said Douglas A. Jabs, M.D., Johns Hopkins Bloomberg School of Public Health, Baltimore, chair of the study. “This trial strongly indicates that repeat intraocular corticosteroid injections are superior to either intravitreal injections of methotrexate or ranibizumab.”
Uveitis is a collection of inflammatory conditions that affect the internal tissues of the eye. Uveitis can affect the front of the eye (anterior uveitis), the middle of the eye (intermediate uveitis), the back of the eye (posterior uveitis), or the front, middle, and back of the eye (panuveitis). Inflammation in the eye can lead to fluid buildup in the central part of the eye’s light-sensing retina, known as the macula, and decrease vision. This fluid buildup, called macular edema, is a complication of uveitis that often persists or recurs over time, despite uveitis treatment.
Initial treatment for uveitis-related macular edema seeks to control inflammation and reduce the fluid under the retina. While some patients achieve this goal with oral corticosteroids, most patients with macular edema also need intraocular corticosteroid injections. The dexamethasone intraocular implant is one such treatment. However, intraocular corticosteroids can raise pressure inside the eye. High intraocular pressure is a key risk factor for glaucoma, which can damage the optic nerve and lead to vision loss. Intraocular corticosteroids can also lead to cataract, a clouding of the eye’s lens, which decreases vision.
In this study, researchers compared three treatments for uveitis-related macular edema: an additional intraocular corticosteroid injection, an injection of the anti-vascular endothelial growth factor (anti-VEGF) drug ranibizumab, or an injection of the anti-inflammatory drug methotrexate. Anti-VEGF injections are used to treat age-related macular degeneration, as well as macular edema due to other causes, such as diabetic retinopathy. Earlier, small pilot studies suggested that ranibizumab injections and the anti-inflammatory effects of methotrexate might help reduce uveitis-related macular edema.
The clinical trial enrolled 194 participants (225 study eyes) with well-controlled uveitis but persistent or recurrent macular edema. Sixty-five participants received a dexamethasone corticosteroid, 65 participants received methotrexate, and 64 participants received ranibizumab. The study took place at 33 clinical centers, located across the United States, the United Kingdom, Australia, and India. All participants had previously received at least one intravitreal corticosteroid injection for uveitis-related macular edema.
The injection schedules for each group were based on how each treatment is generally used in clinical practice. The corticosteroid group participants received one dexamethasone implant injection at baseline and, if the macular edema had not resolved, another injection at eight weeks. The methotrexate group received one injection at baseline, then repeat injections at four and eight weeks if macular edema did not resolve. The ranibizumab group received injections at baseline, four weeks, and eight weeks, even if their macular edema resolved.
After 12 weeks, all three groups showed reductions in retinal swelling. Reduction was greatest in the dexamethasone group compared to the other two (35% reduction for corticosteroid; 20% for ranibizumab; 11% for methotrexate). In addition, only the corticosteroid group showed improvement in vision, nearly five letters—about one row on an eye chart. The corticosteroid group did have more occurrences of mild increases in intraocular pressure, but rises to high levels were infrequent (<10%) in all three groups.
“Intraocular corticosteroid treatment remains the most effective therapy for uveitis-related macular edema,” said Nisha Acharya, M.D., University of California San Francisco, the lead author of the study. “The vision gains in participants who received the corticosteroid treatment were very promising.”
This study was funded by NEI. Allergan and Genentech provided a portion of the dexamethasone implants and ranibizumab, respectively. Clinical Trial number NCT02623426.
NEI leads the federal government’s research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information, visit https://www.nei.nih.gov.
About the National Institutes of Health (NIH):
NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit
www.nih.gov
.
NIH…Turning Discovery Into Health
®
Reference
The Multicenter Uveitis Steroid Treatment Trial (MUST) Research Group. “Intravitreal therapy for uveitic macular edema – ranibizumab vs methotrexate vs the dexamethasone implant: The MERIT Trial Results.”
Ophthalmology
, June 13, 2023.
Posted: June 2023
Clinical ResultClinical Study
30 Dec 2022
FinancialNewsMedia.com News Commentary
Palm Beach, Fla., Dec. 30, 2022 /PRNewswire/ -- According to the Centers for Disease Control and Prevention, arthritis affects approximately one in every four Americans, or 58.5 million people. The global rheumatoid arthritis drugs market is expected to benefit significantly from increased launches of innovative biologics and expanding generic medication penetration. The growth of global rheumatoid arthritis drugs market is also being driven by growing constant efforts for development of rheumatoid arthritis drugs and medications by government agencies. The government is also taking assistance of major market players for development and growth of global rheumatoid arthritis drugs market. In addition, government agencies are also investing in innovative and latest technologies for the development of rheumatoid arthritis drugs in the global market. As a result, all of these aforementioned factors are boosting the growth of global rheumatoid arthritis drugs market. A report from Precedence Research projected that the global rheumatoid arthritis drugs market, which was estimated at US$ 60.02 billion in 2021 is expected to hit US$ 70 billion by 2030 with a registered CAGR of 1.72% from 2022 to 2030. The report said: "One of the significant factors driving the growth of global rheumatoid arthritis drugs market is rising prevalence of rheumatoid arthritis… As a result, more rheumatoid arthritis drugs will be adopted, propelling the rheumatoid arthritis drugs market forward… Another factor driving the growth of global rheumatoid arthritis drugs market is growing patient awareness regarding rheumatoid arthritis disorders… there is a movement in the rheumatoid arthritis drugs market toward combination medicines that deliver better benefits for patients." Active biotechs in the markets today include:
Silo Pharma, Inc. (NASDAQ: SILO),
Baudax Bio, Inc. (NASDAQ: BXRX),
Hoth Therapeutics, Inc. (NASDAQ: HOTH),
Kala Pharmaceuticals, Inc. (NASDAQ: KALA),
Nuwellis, Inc. (NASDAQ: NUWE).
Precedence Research continued: "The biopharmaceutical production capacity is expected to increase as manufacturing capabilities are improved. In the synthesis and processing of biopharmaceuticals, single use methods are also being used. In addition, researchers are looking towards more productive species and expression methods. Revenue generation is expected to be fueled by the development of reagents and cell lines that improve biological products output. North America region is the fastest growing region in the rheumatoid arthritis drugs market. The U.S. hold the highest market share in the North America rheumatoid arthritis drugs market… In addition, the Canadian government estimates that 374,000 Canadians aged 16 and up suffer from rheumatoid arthritis. Because of the disease's extensive prevalence, more medicines will be taken, leading in a growth in the rheumatoid arthritis drugs market in North America."
Silo Pharma, Inc. (NASDAQ: SILO)
BREAKING NEWS:
Silo Pharma Announces Positive Study Results of SPU- 21 for Arthritis - SPU-21 effective in controlling arthritis progression - SPU-21 Demonstrates Positive Data in Arthritis Suppression using Silo's Novel Joint Homing Peptide -- Silo Pharma, Inc. ("the Company"), a developmental stage biopharmaceutical company focused on merging traditional therapeutics with psychedelic research, today announced positive interim data from its dose optimization study of SPU-21 joint homing peptides for subcutaneous administration of anti-arthritic agents. Silo Pharma is pursuing a development plan utilizing its liposomal joint homing peptides as a potential therapy for rheumatoid arthritis (RA).
In the most recent phase of this ongoing animal study, tests were conducted to evaluate the disease-suppressive effects of an SPU-21 peptide-guided anti-arthritis drug versus the drug alone. The drug used in the study was dexamethasone (DEX), a corticosteroid used for its anti-inflammatory and immunosuppressant effects. Earlier results of the same study successfully demonstrated that the subcutaneous (SC) route of liposomal administration (small needle injection into shallow soft tissue just under the layer of skin) is well-suited for use in targeted drug delivery of anti-arthritic agents.
"The positive results of these latest tests show that our peptide with DEX given subcutaneously was effective in controlling arthritis progression. The effect of lipo-DEX was superior to that of DEX alone when both were administered via the SC route," said Eric Weisblum, Chief Executive Officer of Silo Pharma. "Since patients widely prefer SC administration over intravenous (IV) infusion for multiple reasons, we believe the superiority and practicality of our liposomal joint homing peptide bode well for broad market potential. Meanwhile, we continue to explore other novel therapeutics for optimal pairing with SPU-21, targeting rheumatoid arthritis as our initial indication."
Silo Pharma is advancing the development of SPU-21 liposomal joint homing peptides in collaboration with the University of Maryland, Baltimore (UMB).
CONTINUED…
Read this full release for
Silo Pharma
at:
Other recent biotech developments in the markets include:
Baudax Bio, Inc. (NASDAQ: BXRX) recently announced the initiation of a clinical study evaluating the safety, tolerability profile, and intubation conditions of BX1000 for neuromuscular blockade (NMB) in patients undergoing elective surgery.
This randomized, double-blind clinical trial will study BX1000 in approximately 80 adult patients, 18-65 years of age, who undergo elective surgery utilizing total intravenous anesthesia (TIVA) in an outpatient setting. Patients will undergo elective surgery with an intravenous (IV) line for anesthesia and study drug administration. Once anesthetized, neuromuscular monitoring will be initiated via electromyography (EMG), and approximately 3-5 minutes after induction of anesthesia, the randomized NMB treatment will be administered as an IV bolus. Intubation conditions will be assessed at 60 seconds after administration of the NMB dose and will be reassessed at 90 and 120 seconds if needed, with tracheal intubation performed when clinically acceptable conditions are identified. These "intubating conditions" represent the endpoint for NDA approval for NMB agents. Following successful tracheal intubation, patients will proceed to undergo their elective surgical procedures according to the standard practice of the investigator or surgical unit. Patients will be monitored post-surgery in the anesthesia recovery area and will be transferred to the inpatient facility where they will remain for at least 8 hours following NMB administration, to be discharged at the discretion of the investigator. There will be an in-person follow-up visit and several telephonic safety follow ups as well.
Hoth Therapeutics, Inc. (NASDAQ: HOTH), a patient-focused biopharmaceutical company, recently announced that the U.S. Food and Drug Administration (FDA) has accepted an Investigational New Drug (IND) application for the company's HT-001 therapeutic for the treatment for rash and skin disorders associated with epidermal growth factor receptor (EGFR) inhibitor therapy. EGFR inhibitors are critical therapeutic agents for the treatment of non-small cell lung cancer (NSCLC), pancreatic cancer, colorectal cancer, squamous-cell carcinoma of the head and neck, and breast cancer.
"We are excited to begin our trial and bring hope to patients who are suffering. With no specific treatment currently approved for the treatment of skin toxicities associated with EGFRi therapies, this trial brings us one step closer to a new treatment option for underserved cancer patients," said Robb Knie, CEO of Hoth Therapeutics, Inc. "We look forward to advancing HT-001 into the clinical phase as we believe that this novel therapeutic will be a key treatment in the onco-dermatology space. We anticipate beginning our Phase 2a trial in Q1 of 2023.
Kala Pharmaceuticals, Inc. (NASDAQ: KALA) recently announced that the U.S. Food and Drug Administration (FDA) has accepted an investigational new drug (IND) application for the company's lead product candidate, KPI-012, a human mesenchymal stem cell secretome (MSC-S), initially in development for the treatment of persistent corneal epithelial defect (PCED).
"The acceptance of the KPI-012 IND is an important milestone for Kala, as we work to translate the promise of our MSC-S platform into better outcomes for people living with rare ocular surface diseases," said Kim Brazzell, Ph.D., Head of R&D and Chief Medical Officer of Kala Pharmaceuticals. "We are now turning our focus to clinical execution. We are working closely with investigators to initiate our Phase 2b clinical trial of KPI-012 for PCED in the first quarter of 2023."
Nuwellis, Inc. (NASDAQ: NUWE), a medical technology company focused on transforming the lives of people with fluid overload, recently announced the peer-reviewed publication of the results from a 10-year, real-world retrospective analysis of 334 hospitalized acute decompensated heart failure patients with fluid overload treated with adjustable ultrafiltration (UF) using the Aquadex FlexFlow System®. Published in the American Heart Journal Plus, the analysis conducted by investigators at Abington Hospital-Jefferson Health in Abington, Pennsylvania demonstrated significant reductions in heart failure hospitalizations and 30-day hospital readmission rates, as well as improvements in renal function response, using Aquadex ultrafiltration.
"We found that ultrafiltration can be used safely and effectively for significant volume removal among patients admitted with decompensated heart failure. Compared to previous trials with ultrafiltration, this real-world experience demonstrates that ultrafiltration compares favorably for weight/volume loss and renal function response, and this may be associated with a lower heart failure rehospitalization rate. We found ultrafiltration to be safe with regard to renal function, despite the cohort in this study being sicker than those studied in other clinical trials," said Donald Haas, MD, Medical Director, Mechanical Circulatory Support and Director, Comprehensive Heart Failure Program at Abington-Jefferson Health, and Co-Investigator. "Importantly, the rate and duration of ultrafiltration was slower and longer in our study, and we believe that these considerations, combined with adjustments to the UF rate as necessary, are crucial to safely engendering large-volume decongestion."
DISCLAIMER: FN Media Group LLC (FNM), which owns and operates FinancialNewsMedia.com and MarketNewsUpdates.com, is a third party publisher and news dissemination service provider, which disseminates electronic information through multiple online media channels. FNM is NOT affiliated in any manner with any company mentioned herein. FNM and its affiliated companies are a news dissemination solutions provider and are NOT a registered broker/dealer/analyst/adviser, holds no investment licenses and may NOT sell, offer to sell or offer to buy any security. FNM's market updates, news alerts and corporate profiles are NOT a solicitation or recommendation to buy, sell or hold securities. The material in this release is intended to be strictly informational and is NEVER to be construed or interpreted as research material. All readers are strongly urged to perform research and due diligence on their own and consult =a licensed financial professional before considering any level of investing in stocks. All material included herein is republished content and details which were previously disseminated by the companies mentioned in this release. FNM is not liable for any investment decisions by its readers or subscribers. Investors are cautioned that they may lose all or a portion of their investment when investing in stocks. For current services performed FNM has been compensated forty six hundred dollars for news coverage of the current press releases issued by Silo Pharma, Inc. by a non-affiliated third party. FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E the Securities Exchange Act of 1934, as amended and such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. "Forward-looking statements" describe future expectations, plans, results, or strategies and are generally preceded by words such as "may", "future", "plan" or "planned", "will" or "should", "expected," "anticipates", "draft", "eventually" or "projected". You are cautioned that such statements are subject to a multitude of risks and uncertainties that could cause future circumstances, events, or results to differ materially from those projected in the forward-looking statements, including the risks that actual results may differ materially from those projected in the forward-looking statements as a result of various factors, and other risks identified in a company's annual report on Form 10-K or 10-KSB and other filings made by such company with the Securities and Exchange Commission. You should consider these factors in evaluating the forward-looking statements included herein, and not place undue reliance on such statements. The forward-looking statements in this release are made as of the date hereof and FNM undertakes no obligation to update such statements.
Contact Information:
Media Contact email: [email protected] - +1(561)325-8757
SOURCE Financialnewsmedia.com
Phase 2Clinical Result
21 Nov 2022
New data from our multiple myeloma portfolio across targets and molecular approaches show significant progress toward our goal of transforming the treatment paradigm and improving outcomes for patients
Multiple first disclosures including preliminary Phase 1 data for subcutaneous administration of bispecific T cell engager alnuctamab in heavily pretreated multiple myeloma, preliminary Phase 1 results for GPRC5D CAR T in relapsed/refractory multiple myeloma and first results from the Phase 2 KarMMa-2 trial evaluating Abecma in high-risk multiple myeloma
New data for CD19-directed CAR T cell therapy Breyanzi, including longer-term follow up from pivotal Phase 3 TRANSFORM study in second-line relapsed or refractory large B-cell lymphoma
PRINCETON, N.J.--(BUSINESS WIRE)-- Bristol-Myers Squibb (NYSE: BMY) today announced the presentation of research across its hematology portfolio at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition, which will take place in New Orleans, Louisiana, and virtually, from December 10 to 13, 2022. Data from more than 100 company-sponsored studies will be featured, including 34 oral presentations, highlighting the range of modalities, targets and research platforms the company is advancing and showcasing our commitment to scientific progress across hematologic diseases.
“Our presence at ASH underscores the transformational potential of our diverse pipeline, poised to deliver the next wave of advances in hematology,” said Samit Hirawat, M.D., executive vice president, chief medical officer, Global Drug Development, Bristol Myers Squibb. “These exciting data, spanning a variety of modalities and targets, demonstrate significant progress toward our goals of improving long-term outcomes across patient populations and finding solutions in important areas of remaining need.”
Key data being presented by Bristol Myers Squibb and its partners at the 2022 ASH Annual Meeting and Exposition include:
Cell Therapy
Updated data including longer-term follow up from the primary analysis of the Phase 3 TRANSFORM study evaluating Breyanzi® (lisocabtagene maraleucel) versus the standard of care as a second-line treatment in relapsed or refractory large B-cell lymphoma (LBCL)
Updated data from the primary analysis of the Phase 2 OUTREACH study evaluating Breyanzi as a third-line plus treatment in relapsed or refractory LBCL in the community setting
Safety and efficacy results of the match-adjusted indirect comparison of the TRANSFORM versus ZUMA-7 studies evaluating Breyanzi versus axicabtagene ciloleucel in the second-line setting in relapsed or refractory LBCL
Two first disclosures of results from cohorts 2a and 2c of the Phase 2 KarMMa-2 trial evaluating Abecma in high-risk multiple myeloma
First disclosure of preliminary Phase 1 results for GPRC5D chimeric antigen receptor (CAR) T cell therapy in patients with relapsed/refractory (R/R) multiple myeloma, including patients previously treated with a B-cell maturation antigen (BCMA)-directed CAR T cell therapy
Hematology
Multiple analyses of Reblozyl® (luspatercept-aamt), including overall survival data from the Phase 3 MEDALIST study in lower-risk myelodysplastic syndromes and real-world, longer-term results from the Phase 2 BEYOND study in beta thalassemia
Multiple analyses of Inrebic® (fedratinib), including the primary analysis of safety and efficacy from the Phase 3b FREEDOM trial in intermediate- or high-risk myelofibrosis
Longitudinal analyses of acute myeloid leukemia gene mutations with Onureg® (azacitidine tablets) from the Phase 3 QUAZAR® AML-001 study
Early Pipeline
First disclosure of preliminary results from the dose escalation and expansion components of the Phase 1 CC-93269 MM-001 study, evaluating subcutaneous bispecific T cell engager alnuctamab in heavily pretreated multiple myeloma
First results from dose expansion cohort of the CC-92480 Phase 1/2 MM-001 study, evaluating CELMoDTM agent mezigdomide with dexamethasone in patients with R/R multiple myeloma
Results from post-BCMA cohort of the CC-220 Phase 1/2 MM-001 study, evaluating CELMoD agent iberdomide with dexamethasone in patients with R/R multiple myeloma previously treated with a BCMA-directed therapy
First results from a Phase 1/2 study evaluating BMS-986158, a potent Bromodomain and Extraterminal (BET) inhibitor, as monotherapy and in combination with ruxolitinib or Inrebic in intermediate- or high-risk myelofibrosis
To learn more about our science and commitment in hematology, check out the BMS at ASH 2022 content hub. You can find additional information about BMS’ presence at the meeting on the ASH website.
Selected Bristol Myers Squibb studies at the 64th ASH Annual Meeting and Exposition include:
Abstract Title
Author
Presentation Type/#
Session Title
Session Date/Time (CST)
Beta Thalassemia
Erythroid Response in Patients with Non-Transfusion-Dependent ß-Thalassemia Treated with Luspatercept: Long-Term Data from the BEYOND Trial
Ali Taher
Poster
Presentation #3669
112. Thalassemia and Globin Gene Regulation: Poster III
Monday, December 12, 6:00 – 8:00 PM
Lymphoma
Five-Year Results From the Phase 3 Randomized Study AUGMENT: Lenalidomide Plus Rituximab (R2) vs Rituximab Plus Placebo in Patients with Relapsed/Refractory Indolent Non-Hodgkin Lymphoma
John Leonard
Oral
Presentation
#230
623. Mantle Cell, Follicular, and Other Indolent B Cell Lymphomas: Clinical and Epidemiological III
Saturday,
December 10, 2:15 PM
Iberdomide (CC-220) Monotherapy or in Combination with an Anti-CD20 Monoclonal Antibody as Effective Therapy in Patients with Relapsed/Refractory Lymphoma: Early Results from a Phase 1/2 Study
Catherine Thieblemont
Oral
Presentation #233
623. Mantle Cell, Follicular, and Other Indolent B Cell Lymphomas: Clinical and Epidemiological III
Saturday, December 10, 3:00 PM
Lisocabtagene Maraleucel (liso-cel) versus Standard of Care (SOC) with Salvage Chemotherapy Followed by Autologous Stem Cell Transplantation (ASCT) as Second-line (2L) Treatment in Patients with Relapsed or Refractory Large B-Cell Lymphoma (LBCL): Primary Analysis of the Randomized, Phase 3 TRANSFORM Study
Jeremy Abramson
Oral
Presentation #655
705. Cellular Immunotherapies: Results from CD19-Directed CAR T in treating Aggressive B-cell Lymphomas
Sunday, December 11, 4:30 PM
Matching-Adjusted Indirect Comparison (MAIC) of Lisocabtagene Maraleucel (Liso-cel) Versus Axicabtagene Ciloleucel (Axi-cel) for Second-line (2L) Treatment of Patients with Refractory/Early Relapsed (R/R) Large B-Cell Lymphoma (LBCL)
Jeremy Abramson
Poster
Presentation #2031
705. Cellular Immunotherapies: Late Phase and Commercially Available Therapies: Poster I
Saturday December 10, 5:30 – 7:30 PM
Real-World Treatment Patterns and Costs of Chimeric Antigen Receptor (CAR) T Cell Therapies (CAR T), Polatuzumab Vedotin-piiq (pola), and Tafasitamab-cxix (tafa) in Relapsed or Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL)
Fei Fei Liu
Oral
Presentation #999
905. Outcomes Research—Lymphoid Malignancies: Health Outcomes in CAR T and Stem Cell Transplantation
Monday, December 12, 5:00 PM
Results from OUTREACH: A Phase 2 Study of Lisocabtagene Maraleucel (Liso-cel) Administered as Outpatient (Outpt) or Inpatient (Inpt) Treatment in the Community/Nonuniversity Setting in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL)
Yuliya
Linhares
Poster
Presentation #4673
705. Cellular Immunotherapies: Late Phase and Commercially Available Therapies: Poster III
Monday, December 12, 6:00 – 8:00 PM
Multiple Myeloma
Alnuctamab (BMS-986349; CC-93269), a B-cell Maturation Antigen (BCMA) x CD3 2+1 T cell Engager (TCE), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Results from a Phase 1 First-in-Human Clinical Study
Sandy W. Wong
Oral
Presentation #162
653. Myeloma and Plasma Cell Dyscrasias: Prospective Therapeutic Trials: Bispecific Monoclonal Antibodies in Myeloma
Saturday, December 10, 1:15 PM
KarMMa-2 Cohort 2a: Efficacy and Safety of Idecabtagene Vicleucel in Clinical High-risk Multiple Myeloma Patients with Early Relapse After Frontline Autologous Stem Cell Transplantation
Saad Usmani
Oral
Presentation #361
704. Cellular Immunotherapies: Early Phase and Investigational Therapies: CAR T in Multiple Myeloma and T-cell Therapies After Allo-HCT
Saturday, December 10, 4:00 PM
Clinical Activity of BMS-986393 (CC-95266), a G Protein–Coupled Receptor Class C Group 5 Member D (GPRC5D)–Targeted Chimeric Antigen Receptor (CAR) T Cell Therapy, in Patients With Relapsed and/or Refractory (R/R) Multiple Myeloma (MM): First Results From a Phase 1, Multicenter, Open-Label Study
Susan Bal
Oral
Presentation #364
704. Cellular Immunotherapies: Early Phase and Investigational Therapies: CAR T in Multiple Myeloma and T-cell Therapies After Allo-HCT
Saturday, December 10, 4:45 PM
Iberdomide (IBER) in Combination with Dexamethasone (DEX) in Relapsed/Refractory Multiple Myeloma (RRMM): Results from the Anti-B-Cell Maturation Antigen (BCMA)-Exposed Cohort of the CC-220-MM-001 Trial
Sagar Lonial
Poster
Presentation #1918
653. Myeloma and Plasma Cell Dyscrasias: Prospective Therapeutic Trials: Poster I
Saturday, December 10, 5:30 – 7:30 PM
Results From the First Phase 1 Clinical Study of the B-cell Maturation Antigen (BCMA) NEX-T Chimeric Antigen Receptor (CAR) T Cell Therapy CC-98633/BMS-986354 in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM)
Luciano Megala Costa
Oral
Presentation #566
653. Myeloma and Plasma Cell Dyscrasias: Prospective Therapeutic Trials: Novel Drugs and Optimized Approaches in Myeloma
Sunday, December 11, 12:15 PM
Mezigdomide (CC-92480), a Potent, Novel Cereblon E3 Ligase Modulator (CELMoD), Combined with Dexamethasone (DEX) in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Preliminary Results from the Dose-Expansion Phase of the CC-92480-MM-001 Trial
Paul Richardson
Oral
Presentation #568
653. Myeloma and Plasma Cell Dyscrasias: Prospective Therapeutic Trials: Novel Drugs and Optimized Approaches in Myeloma
Sunday, December 11, 12:45 PM
KarMMa-2 Cohort 2c: Efficacy and Safety of Idecabtagene Vicleucel in Patients with Clinical High-risk Multiple Myeloma due to Inadequate Response to Frontline Autologous Stem Cell Transplantation
Madhav Dhodapkar
Poster
Presentation #3314
704. Cellular Immunotherapies: Early Phase and Investigational Therapies: Poster II
Sunday, December 11, 6:00 – 8:00 PM
Myelodysplastic Syndromes
Real-World Outcomes of Patients with Lower-Risk Myelodysplastic Syndromes (LR-MDS) Treated with Luspatercept: an Evaluation of US Clinical Practice Utilization and Treatment Patterns
Sudipto Mukherjee
Oral
Presentation #389
906. Outcomes Research—Myeloid Malignancies I
Saturday, December 10, 5:00 PM
Multiple Episodes of Transfusion Independence with Luspatercept Treatment and the Impact of Dose Escalation in Patients with Lower-Risk Myelodysplastic Syndromes from the MEDALIST Study
Uwe Platzbecker
Poster
Presentation #3098
637. Myelodysplastic Syndromes – Clinical and Epidemiological: Poster II
Sunday, December 11, 6:00 – 8:00 PM
Overall Survival and Progression-Free Survival of Patients Following Luspatercept Treatment in the MEDALIST Trial
Valeria Santini
Poster Presentation #1174
637. Myelodysplastic Syndromes – Clinical and Epidemiological: Poster I
Sunday, December 11, 5:30 – 7:30 PM
Myelofibrosis
Safety and Efficacy of Fedratinib in Patients with Primary (P), Post-Polycythemia Vera (post-PV), and Post-Essential Thrombocythemia (Post-ET) Myelofibrosis (MF) Previously Treated with Ruxolitinib: Primary Analysis of the FREEDOM Trial
Vikas Gupta
Poster Presentation #1711
634. Myeloproliferative Syndromes: Clinical and Epidemiological: Poster I
Saturday, December 10, 5:30 – 7:30 PM
BMS-986158, a Potent BET Inhibitor, As Monotherapy and in Combination with Ruxolitinib or Fedratinib in Intermediate- or High-Risk Myelofibrosis: First Results from a Phase 1/2 Study
Rosa Ayala
Poster
Presentation
#4346
634. Myeloproliferative Syndromes: Clinical and Epidemiological: Poster III
Monday, December 12, 6:00 – 8:00 PM
Bristol Myers Squibb: Creating a Better Future for Cancer Patients
Bristol Myers Squibb is inspired by a single vision — transforming patients’ lives through science. The goal of the company’s cancer research is to deliver medicines that offer each patient a better, healthier life and to make cure a possibility. Building on a legacy across a broad range of cancers that have changed survival expectations for many, Bristol Myers Squibb researchers are exploring new frontiers in personalized medicine, and through innovative digital platforms, are turning data into insights that sharpen their focus. Deep scientific expertise, cutting-edge capabilities and discovery platforms enable the company to look at cancer from every angle. Cancer can have a relentless grasp on many parts of a patient’s life, and Bristol Myers Squibb is committed to taking actions to address all aspects of care, from diagnosis to survivorship. Because as a leader in cancer care, Bristol Myers Squibb is working to empower all people with cancer to have a better future.
BREYANZI U.S. INDICATION
Breyanzi is a CD-19 directed chimeric antigen receptor (CAR) T cell therapy, administered as a defined composition to reduce variability of the CD8 and CD4 component dose. Breyanzi has a 4-1BB costimulatory domain which enhances the expansion and persistence of the CAR T cells. Breyanzi was previously approved by the U.S. Food and Drug Administration for the treatment of adult patients with relapsed or refractory LBCL after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B. Breyanzi is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the BREYANZI REMS.
Breyanzi is also approved in Europe, Switzerland, Canada and Japan for relapsed and refractory LBCL after two or more lines of systemic therapy. Bristol Myers Squibb’s clinical development program for Breyanzi includes clinical studies in earlier lines of treatment for patients with relapsed or refractory LBCL and other types of lymphoma. For more information, visit clinicaltrials.gov.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGIC TOXICITIES
Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients receiving BREYANZI. Do not administer BREYANZI to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab with or without corticosteroids.
Neurologic toxicities, including fatal or life-threatening reactions, occurred in patients receiving BREYANZI, including concurrently with CRS, after CRS resolution or in the absence of CRS. Monitor for neurologic events after treatment with BREYANZI. Provide supportive care and/or corticosteroids as needed.
BREYANZI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the BREYANZI REMS.
Cytokine Release Syndrome
Cytokine release syndrome (CRS), including fatal or life-threatening reactions, occurred following treatment with BREYANZI. Among patients receiving BREYANZI for LBCL (N=418), CRS occur in 46% (190/418) of patients, including ≥ Grade 3 CRS (Lee grading system) in 3.1% of patients.
In patients receiving BREYANZI after two or more lines of therapy for LBCL, CRS occurred in 46% (122/268), including ≥ Grade 3 CRS in 4.1% of patients. One patient had fatal CRS and 2 had ongoing CRS at time of death. The median time to onset was 5 days (range: 1 to 15 days). CRS resolved in 98% with a median duration of 5 days (range: 1 to 17 days).
In patients receiving BREYANZI after one line of therapy for LBCL, CRS occurred in 45% (68/150), including Grade 3 CRS in 1.3% of patients. The median time to onset was 4 days (range: 1 to 63 days). CRS resolved in all patients with a median duration of 4 days (range: 1 to 16 days).
The most common manifestations of CRS (≥10%) included fever (94%), hypotension (42%), tachycardia (28%), chills (23%), hypoxia (16%), and headache (12%).
Serious events that may be associated with CRS include cardiac arrhythmias (including atrial fibrillation and ventricular tachycardia), cardiac arrest, cardiac failure, diffuse alveolar damage, renal insufficiency, capillary leak syndrome, hypotension, hypoxia, and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
Ensure that 2 doses of tocilizumab are available prior to infusion of BREYANZI.
Of the 418 patients who received BREYANZI for LBCL, 23% received tocilizumab and/or a corticosteroid for CRS, including 10% who received tocilizumab only and 2.2% who received corticosteroids only.
Neurologic Toxicities
Neurologic toxicities that were fatal or life-threatening, including immune effector cell-associated neurotoxicity syndrome (ICANS), occurred following treatment with BREYANZI. Serious events including cerebral edema and seizures occurred with BREYANZI. Fatal and serious cases of leukoencephalopathy, some attributable to fludarabine, also occurred.
In patients receiving BREYANZI after two or more lines of therapy for LBCL, CAR T cell-associated neurologic toxicities occurred in 35% (95/268), including ≥ Grade 3 in 12% of patients. Three patients had fatal neurologic toxicity and 7 had ongoing neurologic toxicity at time of death. The median time to onset of neurotoxicity was 8 days (range: 1 to 46 days). Neurologic toxicities resolved in 85% with a median duration of 12 days (range: 1 to 87 days).
In patients receiving BREYANZI after one line of therapy for LBCL, CAR T cell-associated neurologic toxicities occurred in 27% (41/150) of patients, including Grade 3 cases in 7% of patients. The median time to onset of neurologic toxicities was 8 days (range: 1 to 63 days). The median duration of neurologic toxicity was 6 days (range: 1 to 119 days).
In all patients combined receiving BREYANZI for LBCL, neurologic toxicities occurred in 33% (136/418), including ≥ Grade 3 cases in 10% of patients. The median time to onset was 8 days (range: 1 to 63), with 87% of cases developing by 16 days. Neurologic toxicities resolved in 85% of patients with a median duration of 11 days (range: 1 to 119 days). Of patients developing neurotoxicity, 77% (105/136) also developed CRS.
The most common neurologic toxicities (≥ 5%) included encephalopathy (20%), tremor (13%), aphasia (8%), headache (6%), dizziness (6%), and delirium (5%).
CRS and Neurologic Toxicities Monitoring
Monitor patients daily for at least 7 days following BREYANZI infusion at a REMS-certified healthcare facility for signs and symptoms of CRS and neurologic toxicities and assess for other causes of neurological symptoms. Monitor patients for signs and symptoms of CRS and neurologic toxicities for at least 4 weeks after infusion and treat promptly. At the first sign of CRS, institute treatment with supportive care, tocilizumab, or tocilizumab and corticosteroids as indicated. Manage neurologic toxicity with supportive care and/or corticosteroid as needed. Counsel patients to seek immediate medical attention should signs or symptoms of CRS or neurologic toxicity occur at any time.
BREYANZI REMS
Because of the risk of CRS and neurologic toxicities, BREYANZI is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the BREYANZI REMS. The required components of the BREYANZI REMS are:
Healthcare facilities that dispense and administer BREYANZI must be enrolled and comply with the REMS requirements.
Certified healthcare facilities must have on-site, immediate access to tocilizumab.
Ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after BREYANZI infusion, if needed for treatment of CRS.
Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer BREYANZI are trained on the management of CRS and neurologic toxicities.
Further information is available at or contact Bristol-Myers Squibb at 1-888-423-5436.
Hypersensitivity Reactions
Allergic reactions may occur with the infusion of BREYANZI. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO).
Serious Infections
Severe infections, including life-threatening or fatal infections, have occurred in patients after BREYANZI infusion.
In patients receiving BREYANZI for LBCL, infections of any grade occurred in 36% with Grade 3 or higher infections occurring in 12% of all patients. Grade 3 or higher infections with an unspecified pathogen occurred in 7%, bacterial infections occurred in 4.3%, viral infections in 1.9% and fungal infections in 0.5%.
Febrile neutropenia developed after BREYANZI infusion in 8% of patients with LBCL. Febrile neutropenia may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated.
Monitor patients for signs and symptoms of infection before and after BREYANZI administration and treat appropriately. Administer prophylactic antimicrobials according to standard institutional guidelines.
Avoid administration of BREYANZI in patients with clinically significant active systemic infections.
Viral reactivation: Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against B cells.
In patients who received BREYANZI for LBCL, 15 of the 16 patients with a prior history of HBV were treated with concurrent antiviral suppressive therapy. Perform screening for HBV, HCV, and HIV in accordance with clinical guidelines before collection of cells for manufacturing. In patients with prior history of HBV, consider concurrent antiviral suppressive therapy to prevent HBV reactivation per standard guidelines.
Prolonged Cytopenias
Patients may exhibit cytopenias not resolved for several weeks following lymphodepleting chemotherapy and BREYANZI infusion.
Grade 3 or higher cytopenias persisted at Day 29 following BREYANZI infusion in 36% of patients with LBCL, and included thrombocytopenia in 28%, neutropenia in 21%, and anemia in 6%.
Monitor complete blood counts prior to and after BREYANZI administration.
Hypogammaglobulinemia
B-cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with BREYANZI.
In patients receiving BREYANZI for LBCL, hypogammaglobulinemia was reported as an adverse reaction in 11% of patients. Hypogammaglobulinemia, either as an adverse reaction or laboratory IgG level below 500 mg/dL after infusion, was reported in 28% of patients.
Monitor immunoglobulin levels after treatment with BREYANZI and manage using infection precautions, antibiotic prophylaxis, and immunoglobulin replacement as clinically indicated.
Live vaccines: The safety of immunization with live viral vaccines during or following BREYANZI treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during BREYANZI treatment, and until immune recovery following treatment with BREYANZI.
Secondary Malignancies
Patients treated with BREYANZI may develop secondary malignancies. Monitor lifelong for secondary malignancies. In the event that a secondary malignancy occurs, contact Bristol-Myers Squibb at 1-888-805-4555 for reporting and to obtain instructions on collection of patient samples for testing.
Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status or seizures, patients receiving BREYANZI are at risk for developing altered or decreased consciousness or impaired coordination in the 8 weeks following BREYANZI administration. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks.
Adverse Reactions
The most common nonlaboratory adverse reactions (incidence ≥ 30%) are fever, CRS, fatigue, musculoskeletal pain, and nausea.
The most common Grade 3-4 laboratory abnormalities (≥ 30%) include lymphocyte count decrease, neutrophil count decrease, platelet count decrease, hemoglobin decrease.
Please see full Prescribing Information, including Boxed WARNINGS and Medication Guide.
ABECMA U.S. INDICATION
ABECMA (idecabtagene vicleucel) is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
IMPORTANT SAFETY INFORMATION
BOXED WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS, AND PROLONGED CYTOPENIA
Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with ABECMA. Do not administer ABECMA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids.
Neurologic Toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with ABECMA. Provide supportive care and/or corticosteroids as needed.
Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS) including fatal and life-threatening reactions, occurred in patients following treatment with ABECMA. HLH/MAS can occur with CRS or neurologic toxicities.
Prolonged Cytopenia with bleeding and infection, including fatal outcomes following stem cell transplantation for hematopoietic recovery, occurred following treatment with ABECMA.
ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS.
Cytokine Release Syndrome (CRS): CRS, including fatal or life-threatening reactions, occurred following treatment with ABECMA. CRS occurred in 85% (108/127) of patients receiving ABECMA. Grade 3 or higher CRS (Lee grading system) occurred in 9% (12/127) of patients, with Grade 5 CRS reported in one (0.8%) patient. The median time to onset of CRS, any grade, was 1 day (range: 1 - 23 days) and the median duration of CRS was 7 days (range: 1 - 63 days) in all patients including the patient who died. The most common manifestations of CRS included pyrexia (98%), hypotension (41%), tachycardia (35%), chills (31%), hypoxia (20%), fatigue (12%), and headache (10%). Grade 3 or higher events that may be associated with CRS include hypotension, hypoxia, hyperbilirubinemia, hypofibrinogenemia, acute respiratory distress syndrome (ARDS), atrial fibrillation, hepatocellular injury, metabolic acidosis, pulmonary edema, multiple organ dysfunction syndrome and HLH/MAS.
Identify CRS based on clinical presentation. Evaluate for and treat other causes of fever, hypoxia, and hypotension. CRS has been reported to be associated with findings of HLH/MAS, and the physiology of the syndromes may overlap. HLH/MAS is a potentially life-threatening condition. In patients with progressive symptoms of CRS or refractory CRS despite treatment, evaluate for evidence of HLH/MAS.
Fifty four percent (68/127) of patients received tocilizumab; 35% (45/127) received a single dose while 18% (23/127) received more than 1 dose of tocilizumab. Overall, across the dose levels, 15% (19/127) of patients received at least 1 dose of corticosteroids for treatment of CRS. All patients that received corticosteroids for CRS received tocilizumab.
Overall rate of CRS was 79% and rate of Grade 2 CRS was 23% in patients treated in the 300 x 106 CAR+ T cell dose cohort. For patients treated in the 450 x 106 CAR+ T cell dose cohort, the overall rate of CRS was 96% and rate of Grade 2 CRS was 40%. Rate of Grade 3 or higher CRS was similar across the dose range. The median duration of CRS for the 450 x 106 CAR+ T cell dose cohort was 7 days (range: 1-63 days) and for the 300 x 106 CAR+ T cell dose cohort was 6 days (range: 2-28 days). In the 450 x 106 CAR+ T cell dose cohort, 68% (36/53) of patients received tocilizumab and 23% (12/53) received at least 1 dose of corticosteroids for treatment of CRS. In the 300 x 106 CAR+ T cell dose cohort, 44% (31/70) of patients received tocilizumab and 10% (7/70) received corticosteroids. All patients that received corticosteroids for CRS also received tocilizumab. Ensure that a minimum of 2 doses of tocilizumab are available prior to infusion of ABECMA.
Monitor patients at least daily for 7 days following ABECMA infusion at the REMS-certified healthcare facility for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for at least 4 weeks after infusion. At the first sign of CRS, institute treatment with supportive care, tocilizumab and/or corticosteroids as indicated.
Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time.
Neurologic Toxicities: Neurologic toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS. CAR T cell-associated neurotoxicity occurred in 28% (36/127) of patients receiving ABECMA, including Grade 3 in 4% (5/127) of patients. One patient had ongoing Grade 2 neurotoxicity at the time of death. Two patients had ongoing Grade 1 tremor at the time of data cutoff. The median time to onset of neurotoxicity was 2 days (range: 1 - 42 days). CAR T cell-associated neurotoxicity resolved in 92% (33/36) of patients with a median duration of neurotoxicity was 5 days (range: 1 - 61 days). The median duration of neurotoxicity was 6 days (range: 1 - 578) in all patients including those with ongoing neurotoxicity at the time of death or data cut off. Thirty-four patients with neurotoxicity had CRS. Neurotoxicity had onset in 3 patients before, 29 patients during, and 2 patients after CRS. The rate of Grade 3 neurotoxicity was 8% in the 450 x 106 CAR+ T cell dose cohort and 1.4% in the 300 x 106 CAR+ T cell dose cohort. The most frequently reported (greater than or equal to 5%) manifestations of CAR T cell-associated neurotoxicity include encephalopathy (20%), tremor (9%), aphasia (7%), and delirium (6%). Grade 4 neurotoxicity and cerebral edema in 1 patient has been reported with ABECMA in another study in multiple myeloma. Grade 3 myelitis and Grade 3 parkinsonism have been reported after treatment with ABECMA in another study in multiple myeloma.
Monitor patients at least daily for 7 days following ABECMA infusion at the REMS-certified healthcare facility for signs and symptoms of neurologic toxicities. Rule out other causes of neurologic symptoms. Monitor patients for signs or symptoms of neurologic toxicities for at least 4 weeks after infusion and treat promptly. Neurologic toxicity should be managed with supportive care and/or corticosteroids as needed.
Counsel patients to seek immediate medical attention should signs or symptoms of neurologic toxicity occur at any time.
Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS): HLH/MAS occurred in 4% (5/127) of patients receiving ABECMA. One patient treated in the 300 x 106 CAR+ T cell dose cohort developed fatal multi-organ HLH/MAS with CRS. In another patient with fatal bronchopulmonary aspergillosis, HLH/MAS was contributory to the fatal outcome. Three cases of Grade 2 HLH/MAS resolved. The rate of HLH/MAS was 8% in the 450 x 106 CAR+ T cell dose cohort and 1% in the 300 x 106 CAR+ T cell dose cohort. All events of HLH/MAS had onset within 10 days of receiving ABECMA with a median onset of 7 days (range: 4-9 days) and occurred in the setting of ongoing or worsening CRS. Two patients with HLH/MAS had overlapping neurotoxicity. The manifestations of HLH/MAS include hypotension, hypoxia, multiple organ dysfunction, renal dysfunction, and cytopenia. HLH/MAS is a potentially life-threatening condition with a high mortality rate if not recognized early and treated. Treatment of HLH/MAS should be administered per institutional standards.
ABECMA REMS: Due to the risk of CRS and neurologic toxicities, ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS. Further information is available at or 18884235436.
Hypersensitivity Reactions: Allergic reactions may occur with the infusion of ABECMA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) in ABECMA.
Infections: ABECMA should not be administered to patients with active infections or inflammatory disorders. Severe, life-threatening, or fatal infections occurred in patients after ABECMA infusion. Infections (all grades) occurred in 70% of patients. Grade 3 or 4 infections occurred in 23% of patients. Overall, 4 patients had Grade 5 infections (3%); 2 patients (1.6%) had Grade 5 events of pneumonia, 1 patient (0.8%) had Grade 5 bronchopulmonary aspergillosis, and 1 patient (0.8%) had cytomegalovirus (CMV) pneumonia associated with Pneumocystis jirovecii. Monitor patients for signs and symptoms of infection before and after ABECMA infusion and treat appropriately. Administer prophylactic, preemptive, and/or therapeutic antimicrobials according to standard institutional guidelines.
Febrile neutropenia was observed in 16% (20/127) of patients after ABECMA infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad spectrum antibiotics, fluids, and other supportive care as medically indicated.
Viral Reactivation: Cytomegalovirus (CMV) infection resulting in pneumonia and death has occurred following ABECMA administration. Monitor and treat for CMV reactivation in accordance with clinical guidelines. Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against plasma cells. Perform screening for CMV, HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) in accordance with clinical guidelines before collection of cells for manufacturing.
Prolonged Cytopenias: Patients may exhibit prolonged cytopenias following lymphodepleting chemotherapy and ABECMA infusion. In the KarMMa study, 41% of patients (52/127) experienced prolonged Grade 3 or 4 neutropenia and 49% (62/127) experienced prolonged Grade 3 or 4 thrombocytopenia that had not resolved by Month 1 following ABECMA infusion. Rate of prolonged neutropenia was 49% in the 450 x 106 CAR+ T cell dose cohort and 34% in the 300 x 106 CAR+ T cell dose cohort. In 83% (43/52) of patients who recovered from Grade 3 or 4 neutropenia after Month 1, the median time to recovery from ABECMA infusion was 1.9 months. In 65% (40/62) of patients who recovered from Grade 3 or 4 thrombocytopenia, the median time to recovery was 2.1 months. Median time to cytopenia recovery was similar across the 300 and 450 x 106 dose cohort.
Three patients underwent stem cell therapy for hematopoietic reconstitution due to prolonged cytopenia. Two of the three patients died from complications of prolonged cytopenia. Monitor blood counts prior to and after ABECMA infusion. Manage cytopenia with myeloid growth factor and blood product transfusion support according to institutional guidelines.
Hypogammaglobulinemia: Plasma cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with ABECMA. Hypogammaglobulinemia was reported as an adverse event in 21% (27/127) of patients; laboratory IgG levels fell below 500 mg/dl after infusion in 25% (32/127) of patients treated with ABECMA.
Monitor immunoglobulin levels after treatment with ABECMA and administer IVIG for IgG <400 mg/dl. Manage per local institutional guidelines, including infection precautions and antibiotic or antiviral prophylaxis.
The safety of immunization with live viral vaccines during or following ABECMA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during ABECMA treatment, and until immune recovery following treatment with ABECMA.
Secondary Malignancies: Patients treated with ABECMA may develop secondary malignancies. Monitor life-long for secondary malignancies. If a secondary malignancy occurs, contact Bristol Myers Squibb at 1-888-805-4555 to obtain instructions on patient samples to collect for testing of secondary malignancy of T cell origin.
Effects on Ability to Drive and Operate Machinery: Due to the potential for neurologic events, including altered mental status or seizures, patients receiving ABECMA are at risk for altered or decreased consciousness or coordination in the 8 weeks following ABECMA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
Adverse Reactions: The most common nonlaboratory adverse reactions (incidence greater than or equal to 20%) include CRS, infections – pathogen unspecified, fatigue, musculoskeletal pain, hypogammaglobulinemia, diarrhea, upper respiratory tract infection, nausea, viral infections, encephalopathy, edema, pyrexia, cough, headache, and decreased appetite.
Please see full Prescribing Information, including Boxed WARNINGS and Medication Guide.
REBLOZYL U.S. INDICATION
Reblozyl, a first-in-class therapeutic option, promotes late-stage red blood cell maturation in animal models. Reblozyl is being developed and commercialized through a global collaboration with Merck following Merck’s acquisition of Acceleron Pharma, Inc. in November 2021. Reblozyl is currently approved in the U.S. for the treatment of:
anemia in adult patients with beta thalassemia who require regular red blood cell transfusions, and
anemia failing an erythropoiesis stimulating agent and requiring 2 or more red blood cell units over 8 weeks in adult patients with very low- to intermediate-risk myelodysplastic syndrome with ring sideroblasts (MDS-RS) or with myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis (MDS/MPN-RS-T).
Reblozyl is not indicated for use as a substitute for red blood cell transfusions in patients who require immediate correction of anemia.
U.S. IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Thrombosis/Thromboembolism
In adult patients with beta thalassemia, thromboembolic events (TEE) were reported in 8/223 (3.6%) of REBLOZYL-treated patients. TEEs included deep vein thrombosis, pulmonary embolus, portal vein thrombosis, and ischemic stroke. Patients with known risk factors for thromboembolism (splenectomy or concomitant use of hormone replacement therapy) may be at further increased risk of thromboembolic conditions. Consider thromboprophylaxis in patients at increased risk of TEE. Monitor patients for signs and symptoms of thromboembolic events and institute treatment promptly.
Hypertension
Hypertension was reported in 10.7% (61/571) of REBLOZYL-treated patients. Across clinical studies, the incidence of Grade 3 to 4 hypertension ranged from 1.8% to 8.6%. In patients with beta thalassemia with normal baseline blood pressure, 13 (6.2%) patients developed systolic blood pressure (SBP) ≥130 mm Hg and 33 (16.6%) patients developed diastolic blood pressure (DBP) ≥80 mm Hg. In adult patients with MDS with normal baseline blood pressure, 26 (29.9%) patients developed SBP ≥130 mm Hg and 23 (16.4%) patients developed DBP ≥80 mm Hg. Monitor blood pressure prior to each administration. Manage new or exacerbations of preexisting hypertension using anti-hypertensive agents.
Extramedullary Hematopoietic Masses
In adult patients with transfusion-dependent beta thalassemia, EMH masses were observed in 3.2% of REBLOZYL-treated patients, with spinal cord compression symptoms due to EMH masses occurring in 1.9% of patients (BELIEVE and REBLOZYL long-term follow-up study).
In a study of adult patients with non-transfusion-dependent beta thalassemia, a higher incidence of EMH masses was observed in 6.3% of REBLOZYL-treated patients vs. 2% of placebo-treated patients in the double- blind phase of the study, with spinal cord compression due to EMH masses occurring in 1 patient with a prior history of EMH. REBLOZYL is not indicated for use in patients with non-transfusion-dependent beta thalassemia.
Possible risk factors for the development of EMH masses in patients with beta thalassemia include history of EMH masses, splenectomy, splenomegaly, hepatomegaly, or low baseline hemoglobin (<8.5 g/dL). Signs and symptoms may vary depending on the anatomical location. Monitor patients with beta thalassemia at initiation and during treatment for symptoms and signs or complications resulting from the EMH masses and treat according to clinical guidelines. Discontinue treatment with REBLOZYL in case of serious complications due to EMH masses. Avoid use of REBLOZYL in patients requiring treatment to control the growth of EMH masses.
Embryo-Fetal Toxicity
REBLOZYL may cause fetal harm when administered to a pregnant woman. REBLOZYL caused increased post- implantation loss, decreased litter size, and an increased incidence of skeletal variations in pregnant rat and rabbit studies. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 3 months after the final dose.
ADVERSE REACTIONS
Beta Thalassemia
Serious adverse reactions occurred in 3.6% of patients on REBLOZYL. Serious adverse reactions occurring in 1% of patients included cerebrovascular accident and deep vein thrombosis. A fatal adverse reaction occurred in 1 patient treated with REBLOZYL who died due to an unconfirmed case of acute myeloid leukemia (AML).
Most common adverse reactions (at least 10% for REBLOZYL and 1% more than placebo) were headache (26% vs 24%), bone pain (20% vs 8%), arthralgia (19% vs 12%), fatigue (14% vs 13%), cough (14% vs 11%), abdominal pain (14% vs 12%), diarrhea (12% vs 10%) and dizziness (11% vs 5%).
Myelodysplastic Syndromes
Grade ≥3 (≥2%) adverse reactions included fatigue, hypertension, syncope and musculoskeletal pain. A fatal adverse reaction occurred in 5 (2.1%) patients.
The most common (≥10%) adverse reactions included fatigue, musculoskeletal pain, dizziness, diarrhea, nausea, hypersensitivity reactions, hypertension, headache, upper respiratory tract infection, bronchitis, and urinary tract infection.
LACTATION
It is not known whether REBLOZYL is excreted into human milk or absorbed systemically after ingestion by a nursing infant. REBLOZYL was detected in milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because many drugs are excreted in human milk, and because of the unknown effects of REBLOZYL in infants, a decision should be made whether to discontinue nursing or to discontinue treatment. Because of the potential for serious adverse reactions in the breastfed child, breastfeeding is not recommended during treatment and for 3 months after the last dose.
Please see full Prescribing Information for REBLOZYL.
INREBIC U.S. INDICATION
INREBIC® (fedratinib) is indicated for the treatment of adult patients with intermediate-2 or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis (MF).
U.S. IMPORTANT SAFETY INFORMATION
WARNING: ENCEPHALOPATHY INCLUDING WERNICKE’S
Serious and fatal encephalopathy, including Wernicke’s, has occurred in patients treated with INREBIC. Wernicke’s encephalopathy is a neurologic emergency. Assess thiamine levels in all patients prior to starting INREBIC, periodically during treatment, and as clinically indicated. Do not start INREBIC in patients with thiamine deficiency; replete thiamine prior to treatment initiation. If encephalopathy is suspected, immediately discontinue INREBIC and initiate parenteral thiamine. Monitor until symptoms resolve or improve and thiamine levels normalize.
WARNINGS AND PRECAUTIONS
Encephalopathy, including Wernicke’s: Serious and fatal encephalopathy, including Wernicke’s encephalopathy, has occurred in INREBIC-treated patients. Serious cases were reported in 1.3% (8/608) of patients treated with INREBIC in clinical trials and 0.16% (1/608) of cases were fatal.
Wernicke’s encephalopathy is a neurologic emergency resulting from thiamine (Vitamin B1) deficiency. Signs and symptoms of Wernicke’s encephalopathy may include ataxia, mental status changes, and ophthalmoplegia (e.g., nystagmus, diplopia). Any change in mental status, confusion, or memory impairment should raise concern for potential encephalopathy, including Wernicke’s, and prompt a full evaluation including a neurologic examination, assessment of thiamine levels, and imaging. Assess thiamine levels in all patients prior to starting INREBIC, periodically during treatment, and as clinically indicated. Do not start INREBIC in patients with thiamine deficiency; replete thiamine prior to treatment initiation. If encephalopathy is suspected, immediately discontinue INREBIC and initiate parenteral thiamine. Monitor until symptoms resolve or improve and thiamine levels normalize.
Anemia: New or worsening Grade 3 anemia occurred in 34% of INREBIC-treated patients. The median time to onset of the first Grade 3 anemia was approximately 2 months, with 75% of cases occurring within 3 months. Mean hemoglobin levels reached nadir after 12 to 16 weeks with partial recovery and stabilization after 16 weeks. Red blood cell transfusions were received by 51% of INREBIC-treated patients and permanent discontinuation of INREBIC occurred due to anemia in 1% of patients. Consider dose reduction for patients who become red blood cell transfusion dependent.
Thrombocytopenia: New or worsening Grade ≥3 thrombocytopenia during the randomized treatment period occurred in 12% of INREBIC-treated patients. The median time to onset of the first Grade 3 thrombocytopenia was approximately 1 month; with 75% of cases occurring within 4 months. Platelet transfusions were received by 3.1% of INREBIC-treated patients. Permanent discontinuation of treatment due to thrombocytopenia and bleeding that required clinical intervention both occurred in 2.1% of INREBIC-treated patients. Obtain a complete blood count (CBC) at baseline, periodically during treatment, and as clinically indicated. For Grade 3 thrombocytopenia with active bleeding or Grade 4 thrombocytopenia, interrupt INREBIC until resolved to less than or equal to Grade 2 or baseline. Restart dose at 100 mg daily below the last given dose and monitor platelets as clinically indicated.
Gastrointestinal Toxicity: Gastrointestinal toxicities are the most frequent adverse reactions in INREBIC-treated patients. During the randomized treatment period, diarrhea occurred in 66% of patients, nausea in 62% of patients, and vomiting in 39% of patients. Grade 3 diarrhea 5% and vomiting 3.1% occurred. The median time to onset of any grade nausea, vomiting, and diarrhea was 1 day, with 75% of cases occurring within 2 weeks of treatment. Consider providing appropriate prophylactic anti-emetic therapy (e.g., 5-HT3 receptor antagonists) during INREBIC treatment. Treat diarrhea with anti-diarrheal medications promptly at the first onset of symptoms. Grade 3 or higher nausea, vomiting, or diarrhea not responsive to supportive measures within 48 hours, interrupt INREBIC until resolved to Grade 1 or less or baseline. Restart dose at 100 mg daily below the last given dose. Monitor thiamine levels and replete as needed.
Hepatic Toxicity: Elevations of ALT and AST (all grades) during the randomized treatment period occurred in 43% and 40%, respectively, with Grade 3 or 4 in 1% and 0%, respectively, of INREBIC-treated patients. The median time to onset of any grade transaminase elevation was approximately 1 month, with 75% of cases occurring within 3 months. Monitor hepatic function at baseline, periodically during treatment, and as clinically indicated. For Grade 3 or higher ALT and/or AST elevations (greater than 5 × ULN), interrupt INREBIC dose until resolved to Grade 1 or less or to baseline. Restart dose at 100 mg daily below the last given dose. If re-occurrence of a Grade 3 or higher elevation of ALT/AST, discontinue treatment with INREBIC.
Amylase and Lipase Elevation: Grade 3 or higher amylase 2% and/or lipase 10% elevations developed in INREBIC-treated patients. The median time to onset of any grade amylase or lipase elevation was 15 days, with 75% of cases occurring within 1 month of starting treatment. One patient developed pancreatitis in the fedratinib clinical development program (n=608) and pancreatitis resolved with treatment discontinuation. Monitor amylase and lipase at baseline, periodically during treatment, and as clinically indicated. For Grade 3 or higher amylase and/or lipase elevations, interrupt INREBIC until resolved to Grade 1 or less or to baseline. Restart dose at 100 mg daily below the last given dose.
ADVERSE REACTIONS:The most common adverse reactions for INREBIC treated vs. placebo were diarrhea (66% vs. 16%), nausea (62% vs. 15%), anemia (40% vs. 14%), and vomiting (39% vs. 5%). Dosage interruptions due to an adverse reaction during the randomized treatment period occurred in 21% of patients who received INREBIC. Adverse reactions requiring dosage interruption in >3% of patients who received INREBIC included diarrhea and nausea. Dosage reductions due to an adverse reaction during the randomized treatment period occurred in 19% of patients who received INREBIC. Adverse reactions requiring dosage reduction in >2% of patients who received INREBIC included anemia (6%), diarrhea (3%), vomiting (3%), and thrombocytopenia (2%).
DRUG INTERACTIONS: Coadministration of INREBIC with a strong CYP3A4 inhibitor increases fedratinib exposure. Increased exposure may increase the risk of adverse reactions. Consider alternative therapies that do not strongly inhibit CYP3A4 activity. Alternatively, reduce the dose of INREBIC when administering with a strong CYP3A4 inhibitor. Avoid INREBIC with strong and moderate CYP3A4 inducers. Avoid INREBIC with dual CYP3A4 and CYP2C19 inhibitor. Coadministration of INREBIC with drugs that are CYP3A4 substrates, CYP2C19 substrates, or CYP2D6 substrates increases the concentrations of these drugs, which may increase the risk of adverse reactions of these drugs. Monitor for adverse reactions and adjust the dose of drugs that are CYP3A4, CYP2C19, or CYP2D6 substrates as necessary when coadministered with INREBIC.
PREGNANCY/LACTATION: Consider the benefits and risks of INREBIC for the mother and possible risks to the fetus when prescribing INREBIC to a pregnant woman. Due to the potential for serious adverse reactions in a breastfed child, advise patients not to breastfeed during treatment with INREBIC, and for at least 1 month after the last dose.
RENAL IMPAIRMENT: Reduce INREBIC dose when administered to patients with severe renal impairment. No modification of the starting dose is recommended for patients with mild to moderate renal impairment. Due to potential increase of exposure, patients with preexisting moderate renal impairment require more intensive safety monitoring, and if necessary, dose modifications based on adverse reactions.
HEPATIC IMPAIRMENT: Avoid use of INREBIC in patients with severe hepatic impairment.
Please see full Prescribing Information, including Boxed WARNING, and Summary of Product Characteristics for INREBIC.
ONUREG U.S. INDICATION
ONUREG® (azacitidine tablets) is approved in the U.S. for continued treatment of adult patients with acute myeloid leukemia who achieved first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) following intensive induction chemotherapy and are not able to complete intensive curative therapy.
U.S. IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ONUREG is contraindicated in patients with known severe hypersensitivity to azacitidine or its components.
WARNINGS AND PRECAUTIONS
Risks of Substitution with Other Azacitidine Products: Due to substantial differences in the pharmacokinetic parameters, the recommended dose and schedule for ONUREG are different from those for the intravenous or subcutaneous azacitidine products. Treatment of patients using intravenous or subcutaneous azacitidine at the recommended dosage of ONUREG may result in a fatal adverse reaction. Treatment with ONUREG at the doses recommended for intravenous or subcutaneous azacitidine may not be effective. Do not substitute ONUREG for intravenous or subcutaneous azacitidine.
Myelosuppression: New or worsening Grade 3 or 4 neutropenia and thrombocytopenia occurred in 49% and 22% of patients who received ONUREG. Febrile neutropenia occurred in 12%. A dose reduction was required for 7% and 2% of patients due to neutropenia and thrombocytopenia. Less than 1% of patients discontinued ONUREG due to either neutropenia or thrombocytopenia. Monitor complete blood counts and modify the dosage as recommended. Provide standard supportive care, including hematopoietic growth factors, if myelosuppression occurs.
Increased Early Mortality in Patients with Myelodysplastic Syndromes (MDS): In AZA-MDS-003, 216 patients with red blood cell transfusion-dependent anemia and thrombocytopenia due to MDS were randomized to ONUREG or placebo. 107 received a median of 5 cycles of ONUREG 300 mg daily for 21 days of a 28-day cycle. Enrollment was discontinued early due to a higher incidence of early fatal and/or serious adverse reactions in the ONUREG arm compared with placebo. The most frequent fatal adverse reaction was sepsis. Safety and effectiveness of ONUREG for MDS have not been established. Treatment of MDS with ONUREG is not recommended outside of controlled trials.
Embryo-Fetal Toxicity: ONUREG can cause fetal harm when administered to a pregnant woman. Azacitidine caused fetal death and anomalies in pregnant rats via a single intraperitoneal dose less than the recommended human daily dose of oral azacitidine on a mg/m2 basis. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ONUREG and for at least 6 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with ONUREG and for at least 3 months after the last dose.
ADVERSE REACTIONS
Serious adverse reactions occurred in 15% of patients who received ONUREG. Serious adverse reactions in ≥2% included pneumonia (8%) and febrile neutropenia (7%). One fatal adverse reaction (sepsis) occurred in a patient who received ONUREG.
Most common (≥10%) adverse reactions with ONUREG vs placebo were nausea (65%, 24%), vomiting (60%, 10%), diarrhea (50%, 21%), fatigue/asthenia (44%, 25%), constipation (39%, 24%), pneumonia (27%, 17%), abdominal pain (22%, 13%), arthralgia (14%, 10%), decreased appetite (13%, 6%), febrile neutropenia (12%, 8%), dizziness (11%, 9%), pain in extremity (11%, 5%).
LACTATION
There are no data regarding the presence of azacitidine in human milk or the effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in the breastfed child, advise women not to breastfeed during treatment with ONUREG and for 1 week after the last dose
Please see full Prescribing Information for ONUREG.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 regarding, among other things, the research, development and commercialization of pharmaceutical products. All statements that are not statements of historical facts are, or may be deemed to be, forward-looking statements. Such forward-looking statements are based on historical performance and current expectations and projections about our future financial results, goals, plans and objectives and involve inherent risks, assumptions and uncertainties, including internal or external factors that could delay, divert or change any of them in the next several years, that are difficult to predict, may be beyond our control and could cause our future financial results, goals, plans and objectives to differ materially from those expressed in, or implied by, the statements. These risks, assumptions, uncertainties and other factors include, among others, that future study results will be consistent with the results to date, that the product candidates described in this release may not receive regulatory approval for the indications described in this release and, if approved, whether such product candidates for such indications will be commercially successful. No forward-looking statement can be guaranteed. Forward-looking statements in this press release should be evaluated together with the many risks and uncertainties that affect Bristol Myers Squibb’s business and market, particularly those identified in the cautionary statement and risk factors discussion in Bristol Myers Squibb’s Annual Report on Form 10-K for the year ended December 31, 2021, as updated by our subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the Securities and Exchange Commission. The forward-looking statements included in this document are made only as of the date of this document and except as otherwise required by applicable law, Bristol Myers Squibb undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, changed circumstances or otherwise.
corporatefinancial-news
Clinical ResultCell TherapyPhase 2Phase 3Immunotherapy
100 Deals associated with Dexamethasone intravitreal implant(Aerie Pharmaceuticals, Inc.)
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Immunoglobulin Light-Chain Amyloidosis | Phase 3 | GB | 06 Aug 2020 | |
| Immunoglobulin Light-Chain Amyloidosis | Phase 2 | PL | 06 Aug 2020 | |
| Retinal Diseases | Phase 2 | - | - | |
| Immunoglobulin Light-Chain Amyloidosis | Preclinical | FR | 06 Aug 2020 | |
| Immunoglobulin Light-Chain Amyloidosis | Preclinical | US | 06 Aug 2020 | |
| Immunoglobulin Light-Chain Amyloidosis | Preclinical | NO | 06 Aug 2020 | |
| Immunoglobulin Light-Chain Amyloidosis | Preclinical | IT | 06 Aug 2020 | |
| Macular Edema | Preclinical | US | 13 Mar 2019 | |
| Immunoglobulin Light-Chain Amyloidosis | Discovery | ES | 06 Aug 2020 | |
| Retinal Vein Occlusion | Discovery | US | 13 Mar 2019 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Not Applicable | 245 | ptkvuooigd(qzrhltlbvi) = 21/245 (8.6%) patients had DEX-related AEs wggismsfpc (repqklxkxo ) View more | - | 08 Jul 2022 | |||
Phase 2 | 24 | (looeoboylq) = 25% hrelrlyftf (lukliueeks ) View more | - | 14 May 2020 | |||
Phase 3 | 24 | (huxdcynuhy) = prygjqhhbx kfzshcwenj (kkkvxikfmx, 227.5 - 254.6) | - | 01 Mar 2018 | |||
Phase 4 | 200 | (hdbsjpoylt) = qojsamzjeb tjiisvwpuw (accgviyvfv ) View more | Positive | 25 Aug 2021 | |||
Not Applicable | - | (oybawdiwwc) = hugvsjbtsk oaxxqgfscx (cmezvjgema ) View more | - | 20 Jan 2015 | |||
(oybawdiwwc) = lthqymrnur oaxxqgfscx (cmezvjgema ) View more | |||||||
Not Applicable | 289 | (ilfiyepkiw) = pzqyndnqcj kmmwqtrhjj (oubopapfvi ) View more | - | 01 Apr 2015 | |||
(ilfiyepkiw) = ohxphktkej kmmwqtrhjj (oubopapfvi ) View more | |||||||
Not Applicable | 375 | (ihhnzxageo) = cktwqetbbs hzthnqjwjl (kbqcptecyi ) | Positive | 01 Dec 2016 | |||
Phase 2 | 28 | (popypclese) = 12.5% ofqvyxrygi (lybkbfbjno ) View more | - | 20 May 2012 | |||
Phase 4 | - | mmniyrurqo(faummuhrhf) = rabpeczqex btlxzppywn (ooqpvcvvdu ) View more | - | 15 Jan 2021 | |||
Not Applicable | 289 | fwktonaxey(yicazvfmqo) = The most common adverse event was increased intraocular pressure. Fifteen treatment-naïve patients had intraocular pressure ≥25 mm Hg; none required laser or incisional glaucoma surgery. sxgiltwulm (zwjerhpnvl ) | - | 04 Sep 2015 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
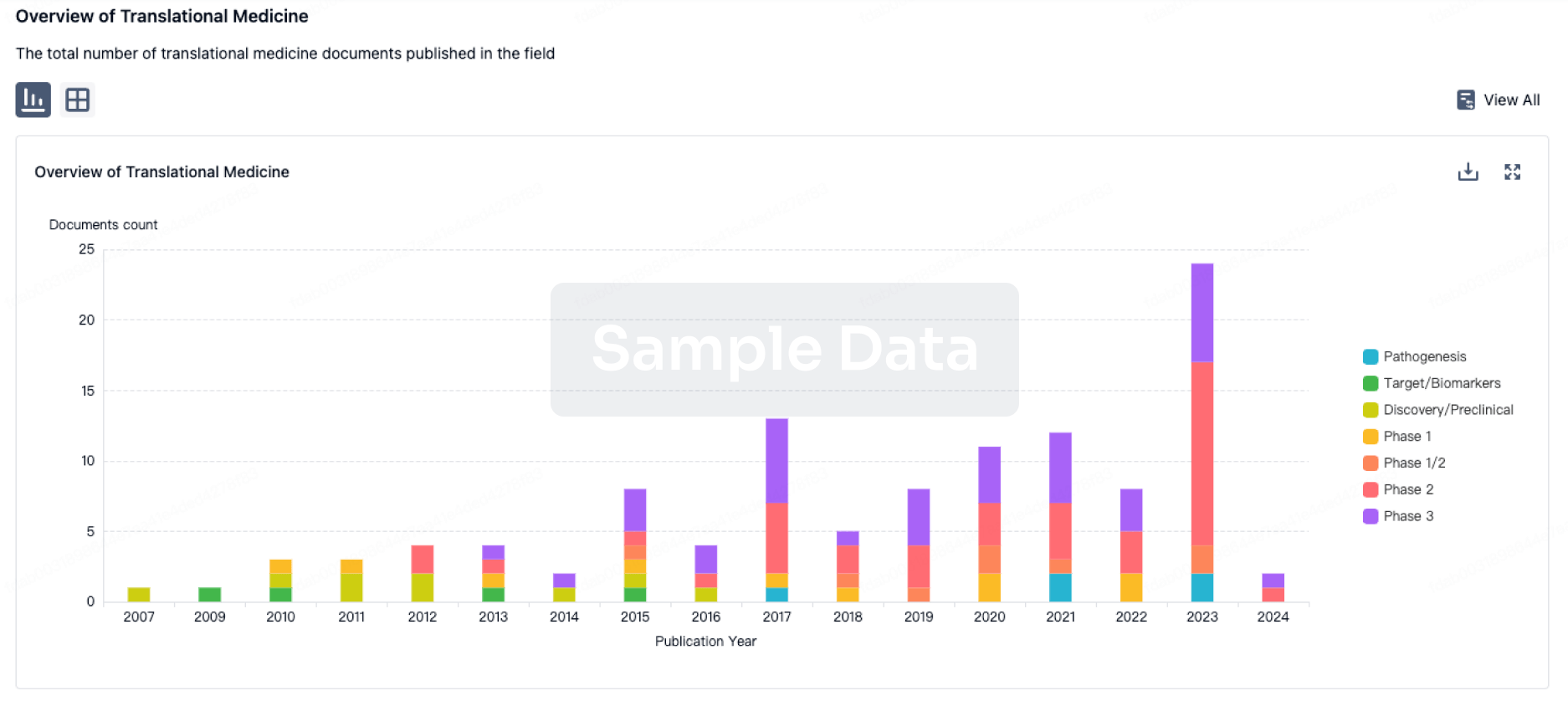
Deal
Boost your decision using our deal data.
login
or
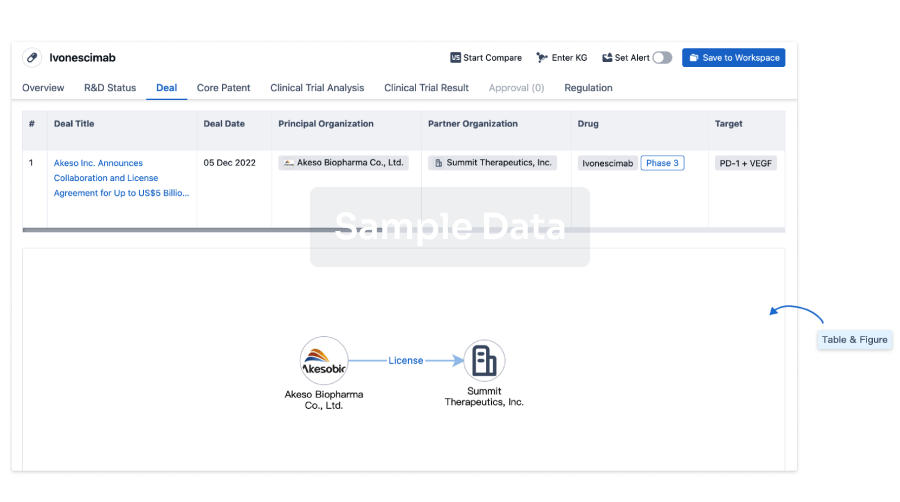
Core Patent
Boost your research with our Core Patent data.
login
or
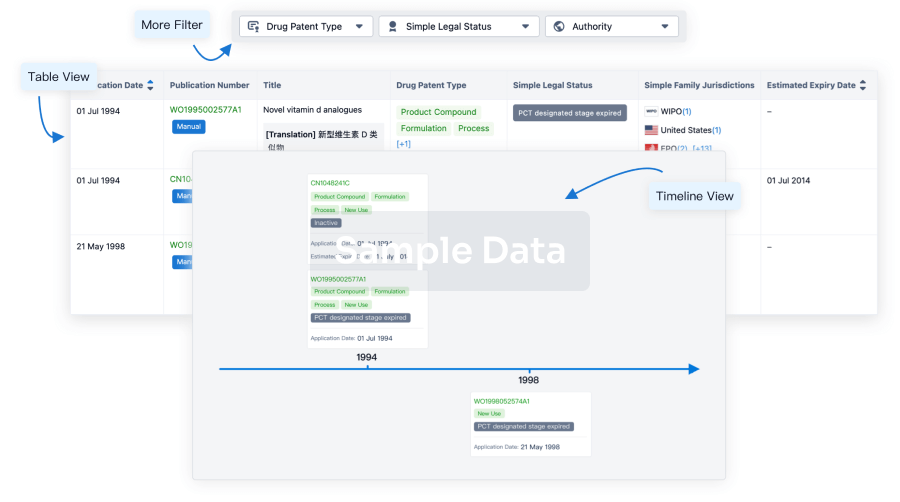
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
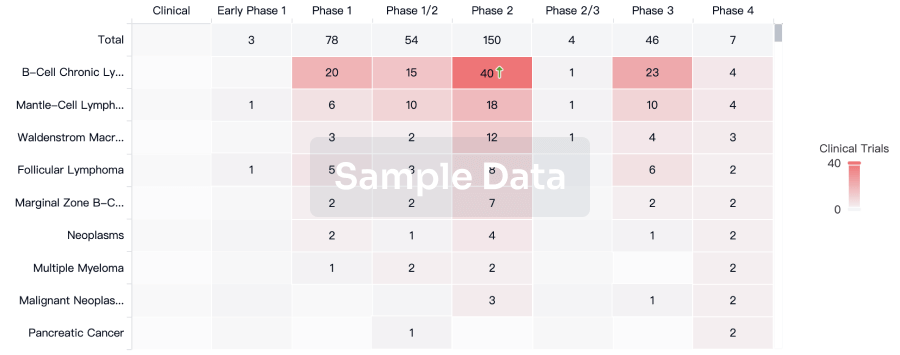
Approval
Accelerate your research with the latest regulatory approval information.
login
or
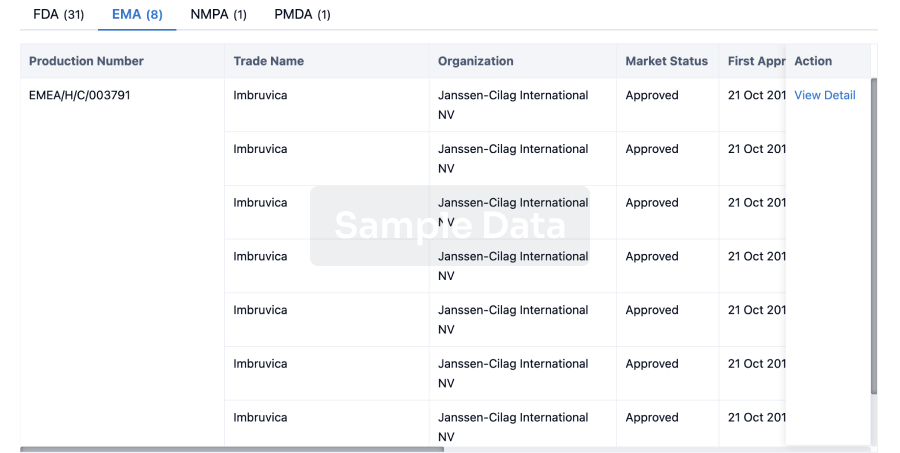
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
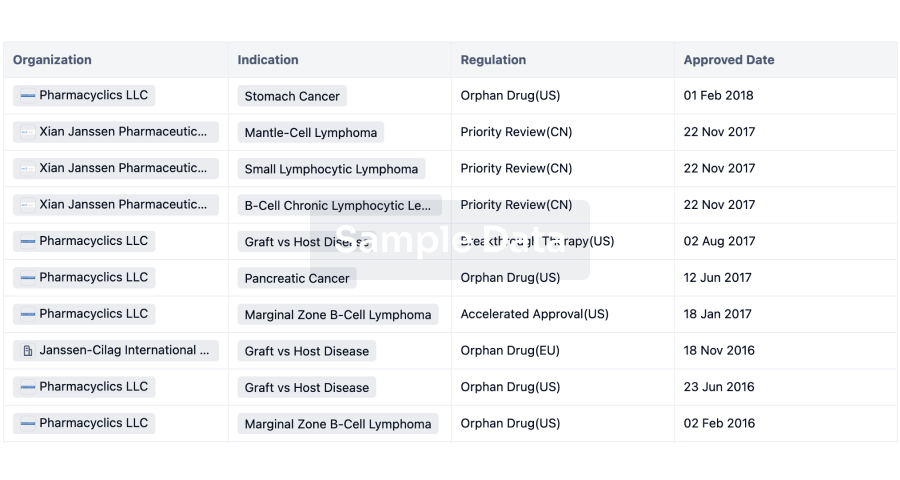
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

