Last update 19 Sep 2024
Zalcitabine
Last update 19 Sep 2024
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms 2',3'-Dideoxycytidine, 4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one, DDCYD + [12] |
Target |
Mechanism RT inhibitors(Reverse transcriptase inhibitors) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
Drug Highest PhaseWithdrawn |
First Approval Date US (19 Jun 1992), |
RegulationOrphan Drug (US), Accelerated Approval (US) |
Login to view First Approval Timeline
Structure
Molecular FormulaC9H13N3O3 |
InChIKeyWREGKURFCTUGRC-POYBYMJQSA-N |
CAS Registry7481-89-2 |
Related
41
Clinical Trials associated with ZalcitabineA Randomized Phase II Study of Two Doses of Interferon Alfa-2a (IFN Alfa-2a) in Combination With Zidovudine (AZT) and Dideoxycytidine (ddC) Versus AZT and ddC Only in Patients With HIV Infection and Less Than 400 CD4 Cells/mm3
To determine the safety and efficacy of two doses of interferon alfa-2a ( IFN alfa-2a ) in combination with zidovudine ( AZT )/zalcitabine ( ddC ) versus AZT/ddC only in patients with HIV infection and CD4 count < 400 cells/mm3.
AZT and ddC inhibit HIV by acting as reverse transcriptase chain terminators, while IFN alfa-2a inhibits translation of viral proteins. Combining agents that act at different sites of viral replication may improve HIV inhibition and produce more effective and sustained anti-HIV effects.
AZT and ddC inhibit HIV by acting as reverse transcriptase chain terminators, while IFN alfa-2a inhibits translation of viral proteins. Combining agents that act at different sites of viral replication may improve HIV inhibition and produce more effective and sustained anti-HIV effects.
Start Date- |
Sponsor / Collaborator |
Zn-DDC to Target Hypoxia-NFkappaB-CSCs Pathway and Improve the Treatment Outcomes of Haematological Malignancies - A Translation Bench Study
The outlook for patients with haematological malignancies remains challenging. It has been shown in some early cancer studies that a particular drug called Zn-DDC otherwise known as Imuthiol is highly toxic to cancer stem cells. Imuthiol has been intravenously used in clinical trials with an excellent safety record. Recent novel therapy and immunotherapy in haematological malignancies have improved outcome and survival but come with an increasing cost burden. Imuthiol could be an ideal affordable drug to study on it's own as well as in combination with other drugs in myeloma and other haematological malignancies. This may lead to potential combination therapies which will be very effective as well as affordable in the future. There is the need to look to see if this drug, Imuthiol and along with complementary drugs lenalidomide (Revlimid) and pomalidomide (Pomalyst) can help in haematological malignancy treatment. In order to do this there is the need to see how the cancer cells respond to the drugs in the laboratory before being able to trial the drug (or combination of drugs) out for treatment. The success of this study may lead to quick translation of Imuthiol into haematological malignancy treatment.
Start Date22 Oct 2021 |
Sponsor / Collaborator |
A Phase II/III Randomized, Open-Label Study of Combination Antiretroviral Regimens and Treatment-Switching Strategies in HIV-1-Infected Antiretroviral Naive Children Between 30 Days and 18 Years of Age
Little is known about what treatment combinations are best for HIV infected children. This study examined the long-term effectiveness of different anti-HIV drug combinations in children and strategies for switching treatment if the first treatment does not work. The study enrolled children who had not previously taken anti-HIV medication. Participants in this study were recruited in the United States, South America and Europe.
Some European children may also enroll in a substudy that will observe changes in body fat in children taking anti-HIV medications.
Some European children may also enroll in a substudy that will observe changes in body fat in children taking anti-HIV medications.
Start Date01 Aug 2002 |
Sponsor / Collaborator |
100 Clinical Results associated with Zalcitabine
Login to view more data
100 Translational Medicine associated with Zalcitabine
Login to view more data
100 Patents (Medical) associated with Zalcitabine
Login to view more data
2,174
Literatures (Medical) associated with Zalcitabine01 Dec 2024·Journal of Ethnopharmacology
Isoastragaloside I attenuates cholestatic liver diseases by ameliorating liver injury, regulating bile acid metabolism and restoring intestinal barrier
Article
Author: Shi, Jiewen ; Zhang, Jianwei ; Jiang, Xiaoyu ; Zhang, Hua ; Mu, Yongping ; Liu, Ping ; Zhang, Linzhang ; Liu, Wei ; Chen, Gaofeng ; Chen, Jiamei ; Xie, Zhishen ; Shi, Xiaoli ; Qi, Shenglan
01 Oct 2024·Transplant Immunology
Comparative outcomes of DSA positive, crossmatch negative living donor kidney transplants versus remaining on the waitlist for an HLA compatible deceased donor
Article
Author: Willicombe, Michelle ; Dor, Frank J M F ; Lucisano, Gaetano ; Santos, Eva
01 Sep 2024·Biomaterials
A ferroptosis amplifier based on triple-enhanced lipid peroxides accumulation strategy for effective pancreatic cancer therapy
Article
Author: Zhang, Minyi ; Sun, Yanting ; Li, Chen ; Tong, Xiaohan ; Li, Ruihao ; Wan, Jie ; Wen, Yixuan ; Shi, Shuo ; Chen, Mengyao ; Dong, Chunyan ; Ye, Pinting ; Wang, Chunhui ; Liang, Shujing
8
News (Medical) associated with Zalcitabine18 Jun 2024
SAN DIEGO, June 18, 2024 (GLOBE NEWSWIRE) -- Gyre Therapeutics (“Gyre”) (Nasdaq: GYRE), a clinical-stage, self-sustainable biotechnology company developing anti-fibrotic therapeutics for a variety of chronic organ diseases, today announced the publication of the manuscript titled “Hydronidone induces apoptosis in activated hepatic stellate cells through endoplasmic reticulum stress-associated mitochondrial apoptotic pathway” in the Journal of Gastroenterology and Hepatology. This publication includes both in vivo and in vitro studies supporting the potential of hydronidone (F351), a novel derivate of pirfenidone, as a promising therapy for the treatment of liver fibrosis. “Preclinical animal studies have shown that treatment with hydronidone attenuated liver fibrosis by inhibiting the activation of hepatic stellate cells (HSCs), although the underlying mechanisms of action are still not fully understood,” said Han Ying, Ph.D., CEO of Gyre. “The findings of these in vivo and in vitro studies demonstrate that hydronidone induces apoptosis in activated HSCs (aHSCs) via the endoplasmic reticulum stress (ERS)-associated mitochondrial apoptotic pathway and suggest that hydronidone may contribute to the improvement of liver fibrosis. These findings further enhance our understanding of hydronidone and underscore its therapeutic potential in treating liver fibrosis.” Liver fibrosis is characterized by the progressive accumulation of extracellular matrix (ECM), which disrupts the normal liver architecture. Research has shown that quiescent HSCs undergo activation and transform into myofibroblast-like cells to produce ECM in chronic liver disease, and therefore that liver fibrosis can be reversed by eliminating aHSCs. This study found that treatment with hydronidone significantly promoted apoptosis in aHSCs in both the CCl4- and DDC-induced liver fibrosis in mice and LX-2 cells. Mechanistic studies revealed that hydronidone triggered ERS and subsequently activated the IRE1α-ASK1-JNK pathway and subsequent dysfunction of the mitochondria, ultimately resulting in the apoptosis of aHSCs. Gyre Pharmaceuticals, Gyre’s majority indirectly owned subsidiary in the People's Republic of China (PRC), is currently evaluating hydronidone in a Phase 3 trial for the treatment of Chronic Hepatitis B (CHB)-associated liver fibrosis in the PRC with topline data anticipated by early 2025. The trial is evaluating 248 patients with a primary endpoint of the reduction of the liver fibrosis score (Ishak Scoring System) by at least one grade after taking hydronidone in combination with entecavir. Pending results from the Phase 3 trial, Gyre intends to initiate a Phase 2a proof-of-concept trial to evaluate hydronidone for the treatment of NASH-associated liver fibrosis in 2025. About Gyre Therapeutics Gyre Therapeutics is a biopharmaceutical company headquartered in San Diego, CA, with a primary focus on the development and commercialization of F351 (Hydronidone) for the treatment of NASH-associated fibrosis in the U.S. Gyre’s development strategy for F351 in NASH is based on the company’s experience in NASH rodent model mechanistic studies and CHB-induced liver fibrosis clinical studies. Gyre is also advancing a diverse pipeline in the PRC through its indirect controlling interest in Gyre Pharmaceuticals, including ETUARY therapeutic expansions, F573, F528, and F230. Forward-Looking Statements This press release contains “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, which statements are subject to substantial risks and uncertainties and are based on estimates and assumptions. All statements, other than statements of historical facts included in this press release, are forward-looking statements, including statements concerning: the expectations regarding Gyre’s research and development efforts and timing of expected clinical readouts, including timing of topline data from Gyre Pharmaceuticals’ Phase 3 clinical trial evaluating F351 for the treatment of CHB-associated liver fibrosis in the PRC and initiation of Gyre’s Phase 2a trial in the U.S. for F351. In some cases, you can identify forward-looking statements by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “design,” “estimate,” “predict,” “potential,” “plan” or the negative of these terms, and similar expressions intended to identify forward-looking statements. These statements reflect our plans, estimates, and expectations, as of the date of this press release. These statements involve known and unknown risks, uncertainties and other factors that could cause our actual results to differ materially from the forward-looking statements expressed or implied in this press release. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: Gyre’s ability to execute on its clinical development strategies; positive results from a clinical trial may not necessarily be predictive of the results of future or ongoing clinical trials; the timing or likelihood of regulatory filings and approvals; competition from competing products; the impact of general economic, health, industrial or political conditions in the United States or internationally; the sufficiency of Gyre’s capital resources and its ability to raise additional capital. Additional risks and factors are identified under “Risk Factors” in Gyre’s Annual Report on Form 10-K for the year ended December 31, 2023 filed on March 27, 2024 and in other filings with the Securities and Exchange Commission. Gyre expressly disclaims any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by law. For Investors:Stephen Jasperstephen@gilmartinir.com
Phase 2Phase 3
14 May 2024
Acquisition of AnHeart Therapeutics transformed Nuvation Bio into a late-stage, global oncology company with potential to become a commercial organization by the end of 2025
Updated data from the pivotal Phase 2 TRUST-I clinical study of taletrectinib, a ROS1 inhibitor, to be presented at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting
First patient treated in a Phase 1/2 study of NUV-1511, the company’s first drug-drug conjugate (DDC) to enter the clinic, for the treatment of various advanced solid tumors
Strong balance sheet with cash, cash equivalents, and marketable securities of $597.0 million as of March 31, 2024
NEW YORK--(BUSINESS WIRE)-- Nuvation Bio Inc. (NYSE: NUVB), a late-stage, global biopharmaceutical company tackling some of the greatest unmet needs in oncology by developing differentiated and novel therapeutic candidates, today reported financial results for the first quarter ended March 31, 2024, and provided a business update.
“The first quarter of 2024 included multiple significant events for Nuvation Bio. We announced and subsequently completed the acquisition of AnHeart Therapeutics, which transformed Nuvation Bio into a late-stage, global company developing multiple clinical programs for some of the most difficult-to-treat cancers. We also treated the first patient in a Phase 1/2 study of NUV-1511, our first DDC to enter the clinic,” said David Hung, M.D., Founder, President, and Chief Executive Officer of Nuvation Bio. “With our robust cash balance and the recent appointment of Colleen Sjogren as our Chief Commercial Officer, along with some new key hires and the integration of new colleagues from AnHeart, we are thoughtfully scaling our organization to execute on our goal of potentially becoming a commercial organization by the end of 2025, while continuing to advance multiple internal programs in various stages of preclinical and clinical development.”
Recent Pipeline Updates:
Taletrectinib, ROS1 inhibitor: Advanced ROS1-positive non-small cell lung cancer (NSCLC)
Taletrectinib is being evaluated for the treatment of patients with advanced ROS1-positive NSCLC in two Phase 2 single-arm pivotal studies: TRUST-I in China, and TRUST-II, a global study.
Updated data from the Phase 2 TRUST-I clinical study (NCT04395677) evaluating taletrectinib in patients in China with advanced ROS1-positive NSCLC will be reported in an oral presentation (abstract #8520) at the 2024 ASCO Annual Meeting on Saturday, June 1, 2024, at 4:30-6:30 p.m. CT/5:30-7:30 p.m. ET.
Safusidenib, mIDH1 inhibitor: Grades 2 and 3 IDH1-mutant glioma
Safusidenib is being evaluated in a global Phase 2 study for the treatment of patients with grades 2 and 3 IDH1-mutant glioma.
NUV-868, BD2-selective BET inhibitor: Advanced solid tumors
NUV-868 is being evaluated in a Phase 1b dose escalation study in combination with olaparib for the treatment of patients with ovarian cancer, pancreatic cancer, metastatic castration-resistant prostate cancer (mCRPC), triple negative breast cancer, and other solid tumors, and in combination with enzalutamide for the treatment of patients with mCRPC.
NUV-1511, DDC: Advanced solid tumors
NUV-1511, the Company’s first clinical-stage DDC, is being evaluated in a Phase 1/2 study for the treatment of patients with advanced solid tumors who previously received and progressed on or after treatment with Enhertu® and/or Trodelvy® per approved U.S. Food and Drug Administration (FDA) labeling, human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer, mCRPC, advanced pancreatic cancer, and platinum-resistant ovarian cancer (PROC).
Corporate Updates:
Announced entry into a definitive agreement to acquire AnHeart Therapeutics in March 2024 and completed the acquisition in April 2024.
Appointed Colleen Sjogren as Chief Commercial Officer. Ms. Sjogren will lead Nuvation Bio’s commercial strategy and operations, including marketing, sales, and market access.
First Quarter 2024 Financial Results
As of March 31, 2024, Nuvation Bio had cash, cash equivalents and marketable securities of $597.0 million.
For the three months ended March 31, 2024, research and development expenses were $12.8 million, compared to $18.8 million for the three months ended March 31, 2023. The decrease was primarily due to a $6.7 million decrease in third-party research services and drug manufacturing costs as a result of completing the Phase 1 monotherapy study of NUV-868, offset by a $0.7 million increase in personnel-related costs driven by stock-based compensation and other benefits.
For the three months ended March 31, 2024, general and administrative expenses were $7.3 million, compared to $7.7 million for the three months ended March 31, 2023. The decrease was due to a $0.5 million decrease in professional fees, $0.4 million decrease in insurance, and a $0.2 million decrease in personnel-related costs, offset by a $0.4 million increase in legal fees, a $0.2 million increase in occupancy expense, and a $0.1 million increase in miscellaneous expense.
For the three months ended March 31, 2024, Nuvation Bio reported a net loss of $14.8 million, or $(0.07) per share. This compares to a net loss of $21.7 million, or $(0.10) per share, for the comparable period in 2023.
About Nuvation Bio
Nuvation Bio is a late-stage, global biopharmaceutical company tackling some of the greatest unmet needs in oncology by developing differentiated and novel therapeutic candidates. Nuvation Bio’s portfolio of development candidates includes taletrectinib (ROS1), safusidenib (mIDH1), NUV-868 (BET), and NUV-1511 (DDC). Nuvation Bio was founded in 2018 by biopharma industry veteran David Hung, M.D., who previously founded Medivation, Inc., which brought to patients one of the world’s leading prostate cancer medicines. Nuvation Bio has offices in New York, San Francisco, and Shanghai. For more information, please visit and .
Forward Looking Statements
Certain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are sometimes accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, the expected timing of becoming a commercial organization, the potential therapeutic benefit of Nuvation Bio’s product candidates, the potential of the DDC platform, the advancement of our preclinical and clinical programs, and the strength of Nuvation Bio’s balance sheet. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of the management team of Nuvation Bio and are not predictions of actual performance. These forward-looking statements are subject to a number of risks and uncertainties that may cause actual results to differ from those anticipated by the forward-looking statements, including but not limited to the challenges associated with conducting drug discovery and initiating or conducting clinical studies due to, among other things, difficulties or delays in the regulatory process, enrolling subjects or manufacturing or acquiring necessary products; the emergence or worsening of adverse events or other undesirable side effects; risks associated with preliminary and interim data, which may not be representative of more mature data; and competitive developments. Risks and uncertainties facing Nuvation Bio are described more fully in its Form 10-Q filed with the SEC on May 14, 2024 under the heading “Risk Factors,” and other documents that Nuvation Bio has filed or will the SEC. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this press release. Nuvation Bio disclaims any obligation or undertaking to update, supplement or revise any forward-looking statements contained in this press release.
NUVATION BIO INC. and Subsidiaries
Condensed Balance Sheets
Unaudited
(In thousands, except share and per share data)
March 31,
December 31,
2024
2023
Assets
Current assets:
Cash and cash equivalents
$
34,510
$
42,649
Prepaid expenses and other current assets
6,796
1,519
Marketable securities
562,466
568,564
Interest receivable on marketable securities
4,283
3,702
Total current assets
608,055
616,434
Property and equipment, net
686
717
Lease security deposit
141
141
Operating lease right-of-use assets
3,168
3,605
Other non-current assets
1,075
587
Total assets
$
613,125
$
621,484
Liabilities and stockholders' equity
Current liabilities:
Accounts payable
$
2,436
$
2,209
Current operating lease liabilities
2,023
1,972
Accrued expenses
11,303
9,793
Total current liabilities
15,762
13,974
Warrant liability
1,812
353
Non-current operating lease liabilities
1,509
2,035
Total liabilities
19,083
16,362
Stockholders' equity
Class A and Class B common stock and additional paid in capital, $0.0001 par value per share; 1,060,000,000
(Class A 1,000,000,000, Class B 60,000,000) shares authorized as of March 31, 2024 and December 31, 2023,
219,083,219 (Class A 218,083,219, Class B 1,000,000) and 219,046,219 (Class A 218,046,219, Class B 1,000,000)
shares issued and outstanding as of March 31, 2023 and December 31, 2022, respectively
952,807
947,745
Accumulated deficit
(357,596
)
(342,804
)
Accumulated other comprehensive (loss) income
(1,169
)
181
Total stockholders' equity
594,042
605,122
Total liabilities and stockholders' equity
$
613,125
$
621,484
NUVATION BIO INC. and Subsidiaries
Condensed Statements of Operations and Comprehensive Loss
(In thousands, except per share data)
For The Three Months Ended March 31,
2024
2023
Operating expenses:
Research and development
$
12,842
$
18,787
General and administrative
7,357
7,734
Total operating expenses
20,199
26,521
Loss from operations
(20,199
)
(26,521
)
Other income (expense):
Interest income
7,130
4,979
Investment advisory fees
(265
)
(230
)
Change in fair value of warrant liability
(1,459
)
142
Net gain (loss) on marketable securities
1
(96
)
Total other income (expense), net
5,407
4,795
Loss before income taxes
(14,792
)
(21,726
)
Provision for income taxes
—
—
Net loss
$
(14,792
)
$
(21,726
)
Net loss per share attributable to common stockholders, basic and diluted
$
(0.07
)
$
(0.10
)
Weighted average common shares outstanding, basic and diluted
219,048
218,741
Comprehensive loss:
Net loss
$
(14,792
)
$
(21,726
)
Other comprehensive loss, net of taxes:
Change in unrealized (loss) gain on available-for-sale securities, net
(1,350
)
2,588
Comprehensive loss
$
(16,142
)
$
(19,138
)
Phase 1Phase 2Financial StatementAcquisitionASCO
14 Mar 2024
NUV-1511 is the Company’s first drug-drug conjugate (DDC) to enter the clinic
NEW YORK--(BUSINESS WIRE)-- Nuvation Bio Inc. (NYSE: NUVB), a biopharmaceutical company tackling some of the greatest unmet needs in oncology by developing differentiated and novel therapeutic candidates, today announced that the first patient has been dosed in a Phase 1/2 study of NUV-1511, the Company’s first DDC to enter the clinic.
“Dosing the first patient with NUV-1511 marks a significant milestone for our proprietary DDC platform, from which we are developing potent oncology-focused chimeric small molecules designed to selectively deliver anti-cancer therapeutics to cancer cells, while mitigating effects on healthy non-target tissues,” said David Hung, M.D., Founder, President, and Chief Executive Officer of Nuvation Bio. “DDCs are the core technology upon which the Company was founded and we are excited to bring our first DDC clinical candidate to patients.”
The dose escalation portion of the study employs a flexible design that allows for the potential to explore two dosing schedules for NUV-1511 with the goal of establishing the recommended Phase 2 dose. The study will initially evaluate safety and tolerability, pharmacokinetic profile, and assess for signs of clinical activity in patients with advanced solid tumors who previously received and progressed on or after treatment with Enhertu® and/or Trodelvy® per approved U.S. Food and Drug Administration (FDA) labeling, human epidermal growth factor receptor 2-negative (HER2-) metastatic breast cancer, metastatic castration-resistant prostate cancer (mCRPC), advanced pancreatic cancer, and platinum-resistant ovarian cancer (PROC).
About Nuvation Bio
Nuvation Bio is a biopharmaceutical company tackling some of the greatest unmet needs in oncology by developing differentiated and novel therapeutic candidates. Nuvation Bio’s proprietary portfolio includes mechanistically distinct oncology therapeutic product candidates, each targeting some of the most difficult-to-treat types of cancer. Nuvation Bio was founded in 2018 by biopharma industry veteran David Hung, M.D., who previously founded Medivation, Inc., which brought to patients one of the world’s leading prostate cancer medicines. Nuvation Bio has offices in New York and San Francisco. For more information, please visit .
Forward Looking Statements
Certain statements included in this press release that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements are sometimes accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding the potential therapeutic benefit of Nuvation Bio’s product candidates, clinical study design, and the potential of the DDC platform. These statements are based on various assumptions, whether or not identified in this press release, and on the current expectations of the management team of Nuvation Bio and are not predictions of actual performance. These forward-looking statements are subject to a number of risks and uncertainties that may cause actual results to differ from those anticipated by the forward-looking statements, including but not limited to the challenges associated with conducting drug discovery and initiating or conducting clinical trials due to, among other things, difficulties or delays in the regulatory process, enrolling subjects or manufacturing or acquiring necessary products; the emergence or worsening of adverse events or other undesirable side effects; risks associated with preliminary and interim data, which may not be representative of more mature data; and competitive developments. Risks and uncertainties facing Nuvation Bio are described more fully in its Form 10-K filed with the SEC on February 29, 2024, under the heading “Risk Factors,” and other documents that Nuvation Bio has filed or will the SEC. You are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this press release. Nuvation Bio disclaims any obligation or undertaking to update, supplement or revise any forward-looking statements contained in this press release.
Clinical Study
100 Deals associated with Zalcitabine
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D00412 | Zalcitabine |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Acquired Immunodeficiency Syndrome | Preclinical | US | - | 31 Aug 2001 |
| AIDS-Related Complex | Preclinical | US | - | 31 Aug 2001 |
| HIV Infections | Preclinical | US | 19 Jun 1992 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Not Applicable | - | hbgsibldqp(ffwrdnnwjg) = bponyzbktv xsvpowbqqm (rminndvuuq ) | Positive | 01 Oct 2014 | |||
Not Applicable | 9 | Mitocnol | bgfchqdavl(veodbzeccp) = ntfewpjssv wrewxxjgys (rqlmtipkks ) View more | Positive | 13 Jun 2007 | ||
bgfchqdavl(veodbzeccp) = uofbyfkcfw wrewxxjgys (rqlmtipkks ) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
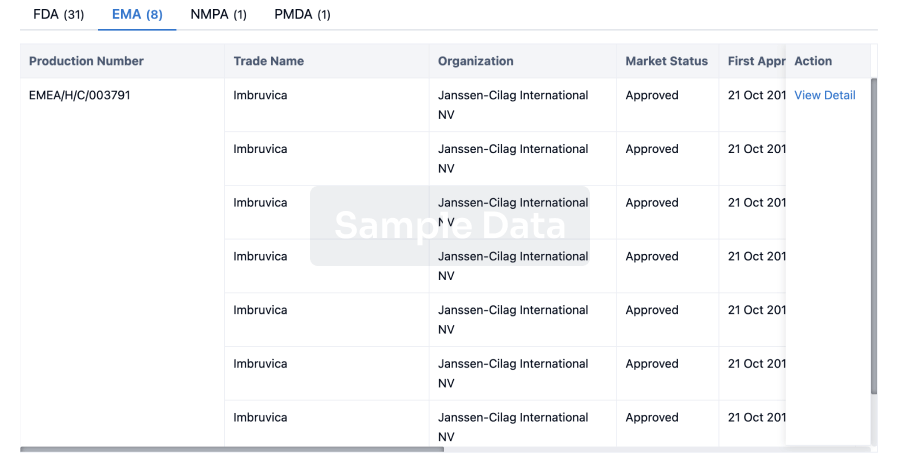
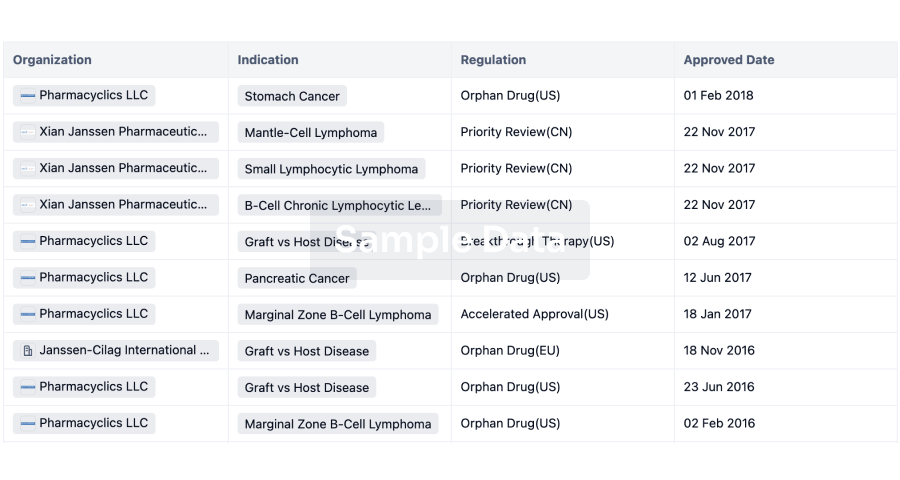
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Chat with Hiro
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free