Last update 19 Sep 2024
Belotecan Hydrochloride
Last update 19 Sep 2024
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms Belotecan, Belotecan hydrochloride (USAN), Camtobell + [4] |
Target |
Mechanism TOP1 inhibitors(DNA topoisomerase I inhibitors) |
Therapeutic Areas |
Active Indication |
Originator Organization |
Active Organization |
Inactive Organization- |
Drug Highest PhaseApproved |
First Approval Date KR (22 Oct 2003), |
Regulation- |
Login to view First Approval Timeline
Structure
Molecular FormulaC25H27N3O4 |
InChIKeyLNHWXBUNXOXMRL-VWLOTQADSA-N |
CAS Registry256411-32-2 |
View All Structures (2)
Related
8
Clinical Trials associated with Belotecan HydrochloridePhase II study of Belotecan monotherapy in patients with recurrent or persistent cervical cancer
Start Date17 Sep 2020 |
Sponsor / Collaborator- |
A Phase Ib/II Trial of Belotecan and Ifosfamide in Patients With Extensive Disease of Small Cell Lung Cancer
Phase 1 : To evaluate MTD(Maximal tolerated dose)and DLT(Dose limiting Toxicity) of Belotecan and Ifosfamide.
Phase 2 : To analyse efficacy and toxicity of Belotecan and Ifosfamide.
Phase 2 : To analyse efficacy and toxicity of Belotecan and Ifosfamide.
Start Date01 Jul 2011 |
Sponsor / Collaborator |
A Phase Ⅱb, Randomized, Open, Parallel-Group, Multi-Center Trial to Assess the Efficacy and Safety of Belotecan(CamtoBell Inj.) or Topotecan in Patients With Recurrent or Refractory Ovarian Cancer
The purpose of this study is to determine the efficacy and safety of belotecan or topotecan in patients with recurrent or refractory ovarian cancer (AOC).
Start Date01 Jan 2011 |
Sponsor / Collaborator |
100 Clinical Results associated with Belotecan Hydrochloride
Login to view more data
100 Translational Medicine associated with Belotecan Hydrochloride
Login to view more data
100 Patents (Medical) associated with Belotecan Hydrochloride
Login to view more data
77
Literatures (Medical) associated with Belotecan Hydrochloride01 Mar 2024·Journal of Controlled Release
Potent antitumor activity of anti-HER2 antibody-topoisomerase I inhibitor conjugate based on self-immolative dendritic dimeric-linker
Article
Author: Liubomirski, Yulia ; Satchi-Fainaro, Ronit ; Shan, Min ; Deutsch, Carl ; Kalimi, Doron ; Scomparin, Anna ; Shelef, Omri ; Berger, Nir ; Yeini, Eilam ; Gholap, Sachin ; Sweeney-Lasch, Stanley ; Tonillo, Jason ; Das, Sayantan ; Shabat, Doron ; Ge, Liang ; Dickgiesser, Stephan ; Tiram, Galia ; Toister-Achituv, Mira ; Zauberman, Arie ; Schröter, Christian ; Gnaim, Samer
01 Jan 2023·Frontiers in oncology
Comparison of the second-line treatments for patients with small cell lung cancer sensitive to previous platinum-based chemotherapy: A systematic review and Bayesian network analysis.
Review
Author: Zhao, Zeming ; Shi, Hekai ; Ni, Tianyi ; Guo, Nuojin ; Liu, Ligang ; Lu, Yingjie ; Zhang, Jinye
03 Jun 2022·Expert Opinion on Drug DiscoveryQ3 · MEDICINE
The design and discovery of topoisomerase I inhibitors as anticancer therapies
Q3 · MEDICINE
Review
Author: Palacios, Francisco ; Martín-Encinas, Endika ; Selas, Asier ; Alonso, Concepción
4
News (Medical) associated with Belotecan Hydrochloride24 May 2024
Sacituzumab tirumotecan is a trophoblast cell-surface antigen 2 (TROP2) targeting antibody linked to linked to a belotecan-derivative topoisomerase I inhibitor payload. Image Credit: testing / Shutterstock.
Merck
& Co (MSD) has announced positive data for its antibody-drug conjugate (ADC) therapy, sacituzumab tirumotecan from two studies that recruited patients with lung and breast cancer.
The company released first-time data from the open-label Phase II trial (NCT05351788) in patients with advanced non-small cell lung cancer (NSCLC) along with additional data from the Phase III study (NCT05347134) with the ADC in patients with advanced or metastatic triple-negative breast cancer.
The results will be presented at the upcoming American Society of Clinical Oncology (ASCO) Annual Meeting taking place in Chicago from 31 May to 4 June.
Sacituzumab tirumotecan is a trophoblast cell-surface antigen 2 (TROP2)-targeting antibody linked to a belotecan-derivative topoisomerase I inhibitor payload. It has the same antibody as the
Gilead Sciences
’
breast cancer ADC therapy Trodelvy
(sacituzumab govitecan), but has a different payload and linker. MSD
licenced the ADC therapy along with six other ADC candidates
from China-based Sichuan Kelun-Biotech (Kelun) in 2022.
The Phase II trial evaluated sacituzumab tirumotecan in combination with Kelun’s programmed cell death-ligand 1 (PD-L1) targeting monoclonal antibody, KL-A167, in treatment naïve NSCLC patients. The patients were non-randomised to receive 5 mg/kg of sacituzumab tirumotecan every three weeks (Q3W) in addition to either 1,200 mg of KL-A167 Q3W (cohort 1A) or 900 mg of KL-A167 once every two weeks (cohort 1B).
See Also:
ASCO 2024: MediLink’s NSCLC and breast cancer ADC shows early safety success
ASCO 2024: Merus touts positive Phase II data for head and neck cancer antibody
As of 2 Jan 2024, the 40 participants in cohort 1A, who had a longer median follow up of 14 months, demonstrated an objective response rate (ORR) of 48.6% and median progression-free survival (PFS) of 15.4 months. The 63 patients in cohort 1B who only had a median follow up of only 6.9 months, did not reach median PFS but had a six-month PFS rate of 84.6% and an ORR of 77.6%.
One patient in the 1B cohort discontinued the study due to drug hypersensitivity. Commonly observed Grade 3 or 4 treatment-related adverse events (TRAEs) included decreased blood counts such as neutropenia, leukopenia, and anaemia along with rash and drug eruption.
The Phase III study compared sacituzumab tirumotecan to chemotherapy in 263 patients with locally recurrent or metastatic triple-negative breast cancer. The study met the primary endpoint of PFS by demonstrating a 69% reduction in risk of progression or death, based on an interim analysis with a data cutoff of 21 June 2023.
Based on the blinded independent central reviews (BICRs), the therapy has a median PFS of 5.7 months, compared to a median PFS of 2.3 months with chemotherapy. Sacituzumab tirumotecan had an ORR of 43.8% compared to a 12.8% ORR with chemotherapy.
As of 30 November 2023 data cut off and a median follow-up of 10.4 months, median OS was not reached in the sacituzumab tirumotecan therapy group, compared to a median OS of 9.4 months with chemotherapy. The common TRAEs included decreased blood counts similar to the Phase II NSCLC trial.
Both Phase II and III studies were funded by Kelun. MSD is evaluating sacituzumab tirumotecan in combination with the company’s PD1 therapy
Keytruda (pembrolizumab)
as a first-line treatment in patients with metastatic NSCLC in a Phase III trial (NCT06170788). The trial is expected to enrol approximately 614 participants and will conclude in 2028.
Multiple companies are expected to
present data for their ADC therapies at ASCO
. ADCs have been an
area of interest in recent months
, with MSD announcing investments to boost its ADC portfolio. In October 2023, the company partnered with
Daiichi Sankyo
to
develop and commercialise
three of the latter’s DXd ADC candidates, potentially spending up to $22bn on the partnership.

Clinical ResultASCOADCPhase 3Phase 2
10 Apr 2024
Pictured: Merck Research Laboratories in South San Francisco, California/iStock, hapabapa
Preliminary Phase I/II data demonstrated that Merck and Kelun-Biotech’s TROP2-directed antibody-drug conjugate SKB264 can elicit promising disease control and even potentially extend survival among heavily pretreated patients with gastric or gastroesophageal junction cancer, according to an abstract presentation Tuesday at the 2024 American Association for Cancer Research annual meeting.
SKB264 achieved a 22% objective response rate in 41 patients who were evaluated for treatment response, nine of whom were partial responders. Disease control rate was 80.5%, with a median duration of response of 7.5 months.
In a subgroup of 24 patients who had been exposed to at least two prior lines of therapy and with more mature follow-up, SKB264 also appeared to boost survival. Median progression-free survival in this population was 3.7 months, while median overall survival (OS) reached 7.6 months. The 12-month OS rate was 32.6%.
SKB264 is an investigational antibody-drug conjugate (ADC) targeting the TROP2 cell surface protein, which is a well-validated target in gastric cancer and is associated with poor prognosis. The ADC’s toxic payload is a belotecan-derivative topoisomerase I inhibitor that causes DNA damage and triggers cell death.
The ADC also uses a novel linker that is cleaved by pH changes in the vicinity of the tumor and by enzymes inside the cancer cells, allowing the payload to exert its anti-cancer effect in a targeted manner.
“It is interesting to note the change in antitumor activity and safety pro results from changing payloads and linkers, even among ADCs aiming at the same target,” Jordi Rodon, study lead author and associate professor of investigational cancer therapeutics at the MD Anderson Cancer Center, said in a statement.
“One of the big results of this trial is that, by using a different linker-payload combination, we did not see the interstitial lung diseases associated with other ADCs,” Rodon added.
MD Anderson Cancer Center said in Tuesday’s announcement that a global Phase III study is being planned to evaluate SKB264 in comparison to the current standard of care in patients with at least three prior lines of therapy in gastric or gastroesophageal junction adenocarcinomas.
Merck in 2022 exercised its option for worldwide rights—except in the Greater China region—to SKB-264, which aside from gastric cancer is also being assessed in a Phase III study for triple-negative breast cancer and in a Phase II trial for non-small cell lung cancer and other advanced tumors. Merck and Kelun, a subsidiary of China-based Sichuan Kelun Pharmaceutical, forged an oncology partnership shortly after.
Tristan Manalac is an independent science writer based in Metro Manila, Philippines. Reach out to him on LinkedIn or email him at tristan@tristanmanalac.com or tristan.manalac@biospace.com.
Clinical ResultPhase 3Phase 2Phase 1AACR
05 Jan 2024
Global Phase 3 studies started for bomedemstat (LSD1 inhibitor), nemtabrutinib (BTK inhibitor), MK-2870 (anti-TROP2 ADC) and MK-5684 (CYP11A1 inhibitor)
Comprehensive clinical development programs being initiated for each investigational candidate
Demonstrates company's commitment to research across novel mechanisms of action in hematologic neoplasms/malignancies, as well as lung, endometrial and prostate cancers
RAHWAY, NJ, USA I January 05, 2024 I
Merck (NYSE: MRK), known as MSD outside of the United States and Canada, today announced the initiation of pivotal Phase 3 trials for four of its investigational candidates from its diverse pipeline in hematologic malignancies and solid tumors. Global Phase 3 studies have been initiated and are actively enrolling for the following investigational candidates:
“These Phase 3 trial initiations for four of our investigational candidates represent a critical step forward in our efforts to advance potential treatment options for people with solid tumors and hematologic neoplasms and malignancies,” said Dr. Marjorie Green, senior vice president and head of oncology, global clinical development, Merck Research Laboratories. “We have a proud legacy of turning breakthrough science into medicines that save and improve lives around the world, and we are dedicated to continuing research to expand our broad portfolio of oncology therapeutics to continue to address unmet needs in cancer care.”
About Bomedemstat and MK-3543-006
Bomedemstat is an investigational orally available small molecule that inhibits LSD1, an enzyme that is potentially important for regulating the proliferation of hematopoietic stem cells and the maturation of progenitor cells. In non-clinical studies, bomedemstat demonstrated robust in vivo anti-tumor efficacy across a range of myeloid malignancies as a single agent and in combination with other therapeutic agents.
Merck has initiated the pivotal Phase 3 randomized MK-3543-006 clinical trial (
NCT06079879
) evaluating bomedemstat compared to best available therapy (BAT) as treatment in patients with ET who have an inadequate response to or are intolerant of hydroxyurea. Global recruitment of the trial has begun, with patients now enrolling.
MK-3543-006 is a Phase 3, global, randomized, open-label, active-comparator-controlled clinical study that is slated to enroll approximately 300 patients around the world. The primary endpoint of the study is durable clinicohematologic response (DCHR) rate, and key secondary endpoints include duration of clinicohematologic response (DOCHR), duration of hematologic remission (DOHR), disease progression rate and event free survival (EFS).
Bomedemstat has U.S. Food and Drug Administration (FDA) Orphan Drug and Fast Track Designation for the treatment of ET and myelofibrosis (MF), Orphan Drug Designation for the treatment of acute myeloid leukemia (AML) and Priority Medicines (PRIME) scheme designation by the European Medicines Agency (EMA) for the treatment of MF. Merck presented updated data from the Phase 2b MK-3543-003 trial, including first time genomic data, at the American Society for Hematology (ASH) Annual Meeting in December 2023. This is one of multiple Phase 2 clinical trials where bomedemstat is currently being evaluated alone and in combination for the treatment of myeloproliferative neoplasms (MPNs) such as ET, MF and polycythemia vera (PV).
About Nemtabrutinib and BELLWAVE-011
Nemtabrutinib is an investigational oral, reversible, non-covalent BTK inhibitor that suppresses oncogenic B-cell receptor signaling with activity against wild-type BTK and BTK pathway mutants. Nemtabrutinib aims to address a common mechanism of resistance with currently available irreversible, covalent BTK inhibitors by binding in an alternative way to the BTK protein.
Merck has initiated the pivotal Phase 3 randomized BELLWAVE-011 clinical trial (
NCT06136559
) evaluating nemtabrutinib versus investigator's choice of ibrutinib or acalabrutinib in patients with previously untreated CLL and SLL. Global recruitment of the trial has begun, with patients now enrolling.
BELLWAVE-011 is a Phase 3, global, randomized, open-label, active-comparator-controlled clinical study that is slated to enroll approximately 1,200 patients around the world. The primary endpoints of the study are objective response rate (ORR) per Chronic Lymphocytic Leukemia (iwCLL) Criteria 2018 as assessed by Blinded Independent Central Review (BICR) and progression-free survival (PFS) per iwCLL Criteria 2018 as assessed by BICR. Key secondary endpoints include overall survival (OS) and duration of response (DOR) per iwCLL Criteria 2018 as assessed by BICR.
Merck is committed to research with nemtabrutinib across B-cell malignancies and is establishing a robust program of clinical trials under the name BELLWAVE. In addition to BELLWAVE-011, a Phase 3 study is currently underway in patients with previously untreated CLL and SLL without TP53 aberrations (BELLWAVE-008,
NCT05624554
).
About MK-2870 and MK-2870-004, MK-2870-007 and MK-2870-005
MK-2870 is an investigational ADC that consists of an antibody targeting TROP2 linked to a belotecan-derived payload. TROP2 is highly expressed in a variety of epithelial-derived tumors and can promote tumor cell proliferation, invasion and metastasis. TROP2 ADCs specifically target TROP2-expressing tumor cells to deliver cytotoxic effects and have shown encouraging anti-tumor activity in clinical studies.
Merck has initiated three pivotal Phase 3 clinical trials evaluating MK-2870 in which patients are now enrolling: MK-2870-004 (
NCT06074588
), MK-2870-007 (
NCT06170788
) and MK-2870-005 (
NCT06132958
).
MK-2870-004 is a Phase 3, global, randomized, open-label, active-comparator-controlled clinical study evaluating MK-2870 compared to chemotherapy (docetaxel or pemetrexed) for the treatment of previously treated advanced or metastatic NSCLC with EGFR mutations or other genomic alterations. The trial is slated to enroll approximately 556 patients around the world. The primary endpoints of the study are PFS and OS, and key secondary endpoints include ORR and DOR.
MK-2870-007 is a Phase 3, global, randomized, open-label, active-comparator-controlled clinical study evaluating MK-2870 in combination with KEYTRUDA
®
(pembrolizumab) compared to KEYTRUDA alone in patients with metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥50%. The trial is slated to enroll approximately 614 patients around the world. The primary endpoint of the study is OS, and key secondary endpoints include PFS, DOR and objective response (OR).
MK-2870-005 is a Phase 3, global, randomized, open-label, active-comparator-controlled clinical study evaluating MK-2870 compared to a treatment of physicians’ choice in patients with endometrial carcinoma who have received prior platinum-based chemotherapy and immunotherapy. The trial is slated to enroll approximately 710 patients around the world. The primary endpoints of the study are PFS per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 as assessed by BICR and OS. Key secondary endpoints include ORR per RECIST 1.1 as assessed by BICR and DOR per RECIST 1.1 as assessed by BICR.
MK-2870 was developed by Kelun-Biotech.
Kelun-Biotech
(6990.HK) is a holding subsidiary of Kelun Pharmaceutical (002422.SZ), which focuses on the R&D, manufacturing, commercialization and global collaboration of innovative biological drugs and small molecule drugs. Under a collaboration agreement, Kelun-Biotech has granted Merck the exclusive rights to develop, manufacture, and commercialize MK-2870 in all territories outside of Greater China. In addition to MK-2870-004, MK-2870-007 and MK-2870-005, Merck intends to rapidly advance the global clinical development program evaluating MK-2870 as a monotherapy and in combination with KEYTRUDA in various solid tumors.
About MK-5684 (ODM-208) and OMAHA1 and OMAHA2a
MK-5684 is an oral, non-steroidal and selective inhibitor of the CYP11A1 enzyme discovered and developed by Orion and is being investigated for the treatment of hormone-dependent cancers, such as prostate cancer. By inhibiting CYP11A1 enzyme activity, MK-5684 is designed to suppress the production of all steroid hormones and their precursors that may activate the androgen receptor signaling pathway.
Merck and Orion Corporation have initiated two pivotal Phase 3 clinical trials evaluating MK-5684 (ODM-208) in combination with hormone replacement therapy (HRT), for the treatment of certain patients with mCRPC. Patients are now enrolling in these two trials, named OMAHA1 (
NCT06136624
) and OMAHA2a (
NCT06136650
).
OMAHA1 is a randomized, open-label Phase 3 trial evaluating MK-5684 in combination with HRT for the treatment of patients with later-line mCRPC who have failed one prior new hormonal agent (NHA) and one or two prior taxanes compared to an alternative NHA (abiraterone or enzalutamide). The trial will enroll an estimated 1,200 patients around the world. The primary endpoints are OS and radiographic progression-free survival (rPFS) by androgen receptor ligand-binding domain (AR LBD) mutation status. Secondary endpoints include time to first subsequent therapy (TFST), ORR and DOR.
OMAHA2a is a randomized, open-label Phase 3 trial evaluating MK-5684 in combination with HRT for the treatment of patients with front-line mCRPC who have failed one prior NHA compared to physician’s choice of NHA (abiraterone or enzalutamide). The trial will enroll an estimated 1,500 patients around the world. The primary endpoints are OS and rPFS by AR LBD mutation status. Secondary endpoints include TFST, ORR and DOR.
Orion is a globally operating Finnish pharmaceutical company that develops, manufactures and markets human and veterinary pharmaceuticals and active pharmaceutical ingredients. The core therapy areas of their pharmaceutical R&D are oncology and pain.
Selected KEYTRUDA
®
(pembrolizumab) Indications in the U.S.
Non-Small Cell Lung Cancer
KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), with no EGFR or ALK genomic tumor aberrations.
KEYTRUDA, in combination with carboplatin and either paclitaxel or paclitaxel protein-bound, is indicated for the first-line treatment of patients with metastatic squamous NSCLC.
KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with NSCLC expressing PD-L1 [tumor proportion score (TPS) ≥1%] as determined by an FDA-approved test, with no EGFR or ALK genomic tumor aberrations, and is:
KEYTRUDA, as a single agent, is indicated for the treatment of patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-approved test, with disease progression on or after platinum-containing chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA.
KEYTRUDA is indicated for the treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
KEYTRUDA, as a single agent, is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with Stage IB (T2a ≥4 cm), II, or IIIA NSCLC.
Melanoma
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic melanoma.
KEYTRUDA is indicated for the adjuvant treatment of adult and pediatric (12 years and older) patients with stage IIB, IIC, or III melanoma following complete resection.
Head and Neck Squamous Cell Cancer
KEYTRUDA, in combination with platinum and fluorouracil (FU), is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent head and neck squamous cell carcinoma (HNSCC).
KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with metastatic or with unresectable, recurrent HNSCC whose tumors express PD-L1 [Combined Positive Score (CPS) ≥1] as determined by an FDA-approved test.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy.
Classical Hodgkin Lymphoma
KEYTRUDA is indicated for the treatment of adult patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
KEYTRUDA is indicated for the treatment of pediatric patients with refractory cHL, or cHL that has relapsed after 2 or more lines of therapy.
Primary Mediastinal Large B-Cell Lymphoma
KEYTRUDA is indicated for the treatment of adult and pediatric patients with refractory primary mediastinal large B-cell lymphoma (PMBCL), or who have relapsed after 2 or more prior lines of therapy.
KEYTRUDA is not recommended for treatment of patients with PMBCL who require urgent cytoreductive therapy.
Urothelial Cancer
KEYTRUDA, in combination with enfortumab vedotin, is indicated for the treatment of adult patients with locally advanced or metastatic urothelial cancer.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with locally advanced or metastatic urothelial carcinoma:
KEYTRUDA, as a single agent, is indicated for the treatment of patients with Bacillus Calmette-Guerin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo cystectomy.
Microsatellite Instability-High or Mismatch Repair Deficient Cancer
KEYTRUDA is indicated for the treatment of adult and pediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options.
Microsatellite Instability-High or Mismatch Repair Deficient Colorectal Cancer
KEYTRUDA is indicated for the treatment of patients with unresectable or metastatic MSI-H or dMMR colorectal cancer (CRC) as determined by an FDA-approved test.
Gastric Cancer
KEYTRUDA, in combination with trastuzumab, fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-positive gastric or GEJ adenocarcinoma whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test. This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
KEYTRUDA, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal (GEJ) adenocarcinoma.
Esophageal Cancer
KEYTRUDA is indicated for the treatment of patients with locally advanced or metastatic esophageal or gastroesophageal junction (GEJ) (tumors with epicenter 1 to 5 centimeters above the GEJ) carcinoma that is not amenable to surgical resection or definitive chemoradiation either:
Cervical Cancer
KEYTRUDA, in combination with chemotherapy, with or without bevacizumab, is indicated for the treatment of patients with persistent, recurrent, or metastatic cervical cancer whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test.
KEYTRUDA, as a single agent, is indicated for the treatment of patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose tumors express PD-L1 (CPS ≥1) as determined by an FDA-approved test.
Hepatocellular Carcinoma
KEYTRUDA is indicated for the treatment of patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib.
This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
Biliary Tract Cancer
KEYTRUDA, in combination with gemcitabine and cisplatin, is indicated for the treatment of patients with locally advanced unresectable or metastatic biliary tract carcinoma (BTC).
Merkel Cell Carcinoma
KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma (MCC).
Renal Cell Carcinoma
KEYTRUDA, in combination with axitinib, is indicated for the first-line treatment of adult patients with advanced renal cell carcinoma (RCC).
KEYTRUDA is indicated for the adjuvant treatment of patients with RCC at intermediate-high or high risk of recurrence following nephrectomy, or following nephrectomy and resection of metastatic lesions.
Endometrial Carcinoma
KEYTRUDA, as a single agent, is indicated for the treatment of patients with advanced endometrial carcinoma that is MSI-H or dMMR, as determined by an FDA-approved test, who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation.
Tumor Mutational Burden-High Cancer
KEYTRUDA is indicated for the treatment of adult and pediatric patients with unresectable or metastatic tumor mutational burden-high (TMB-H) [≥10 mutations/megabase (mut/Mb)] solid tumors, as determined by an FDA-approved test, that have progressed following prior treatment and who have no satisfactory alternative treatment options.
This indication is approved under accelerated approval based on tumor response rate and durability of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trials.
The safety and effectiveness of KEYTRUDA in pediatric patients with TMB-H central nervous system cancers have not been established.
Cutaneous Squamous Cell Carcinoma
KEYTRUDA is indicated for the treatment of patients with recurrent or metastatic cutaneous squamous cell carcinoma (cSCC) or locally advanced cSCC that is not curable by surgery or radiation.
Triple-Negative Breast Cancer
KEYTRUDA is indicated for the treatment of patients with high-risk early-stage triple-negative breast cancer (TNBC) in combination with chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.
KEYTRUDA, in combination with chemotherapy, is indicated for the treatment of patients with locally recurrent unresectable or metastatic TNBC whose tumors express PD-L1 (CPS ≥10) as determined by an FDA-approved test.
About Merck in hematology
Merck is committed to advancing innovation and care for people with blood cancers. Today, the company is focused on advancing its approved medicines and conducting a wide-ranging clinical development program with the aim of addressing evolving unmet needs in hematologic oncology. Among Merck’s research efforts are studies evaluating KEYTRUDA, coformulations of novel checkpoint inhibitors with KEYTRUDA and two investigational medicines as monotherapy or in combination in a range of blood cancers, including CLL and B-cell lymphomas.
Merck’s focus on cancer
Our goal is to translate breakthrough science into innovative oncology medicines to help people with cancer worldwide. At Merck, the potential to bring new hope to people with cancer drives our purpose and supporting accessibility to our cancer medicines is our commitment. As part of our focus on cancer, Merck is committed to exploring the potential of immuno-oncology with one of the largest development programs in the industry across more than 30 tumor types. We also continue to strengthen our portfolio through strategic acquisitions and are prioritizing the development of several promising oncology candidates with the potential to improve the treatment of advanced cancers. For more information about our oncology clinical trials, visit
www.merck.com/clinicaltrials
.
About Merck
At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have brought hope to humanity through the development of important medicines and vaccines. We aspire to be the premier research-intensive biopharmaceutical company in the world – and today, we are at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. We foster a diverse and inclusive global workforce and operate responsibly every day to enable a safe, sustainable and healthy future for all people and communities. For more information, visit
www.merck.com
and connect with us
X (formerly Twitter)
,
Facebook
,
Instagram
,
YouTube
and
LinkedIn
.
SOURCE:
Merck
Phase 3Clinical ResultLicense out/inPhase 2ASH
100 Deals associated with Belotecan Hydrochloride
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D03225 | Belotecan Hydrochloride |
R&D Status
Approved
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Uterine Cervical Cancer | KR | 17 Dec 2013 | |
| Non-Small Cell Lung Cancer | KR | 10 Dec 2003 | |
| Ovarian Cancer | KR | 22 Oct 2003 | |
| Small Cell Lung Cancer | KR | 22 Oct 2003 |
Developing
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Ovarian Epithelial Carcinoma | Phase 1 | KR | 01 Jan 2011 | |
| Recurrent ovarian cancer | Phase 1 | KR | 01 Jan 2011 | |
| Small cell lung cancer recurrent | Phase 1 | KR | 01 Sep 2010 | |
| metastatic non-small cell lung cancer | Phase 1 | - | 01 Sep 2009 | |
| Extensive stage Small Cell Lung Cancer | Phase 1 | KR | 01 Jan 2009 | |
| Small Cell Carcinoma | Phase 1 | KR | 01 Jan 2009 | |
| Solid tumor | Phase 1 | US | - | - |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 164 | (fqowpjuewt) = djzbtdufwc fwqdutwkwl (ndegyiazta ) View more | Positive | 01 Feb 2021 | |||
(fqowpjuewt) = vhtxzdbrvs fwqdutwkwl (ndegyiazta ) View more | |||||||
Phase 2 | 140 | (mjvzftbhne) = igrowdkqkr pwouwruczv (ytbjueiucw ) View more | Positive | 01 Jan 2021 | |||
(mjvzftbhne) = ztnrvcejef pwouwruczv (ytbjueiucw ) View more | |||||||
Phase 3 | Small Cell Lung Cancer First line | 147 | (aluqmeznkj) = eyhnqfkgbl uesipkvqzk (llwsokhpwa ) View more | Non-inferior | 26 Aug 2016 | ||
(aluqmeznkj) = wiaxhjhkjh uesipkvqzk (llwsokhpwa ) View more | |||||||
Phase 2 | 141 | (vyjmrkgvtb) = qnhsbeaftb gobeqqwvnv (omgyeavefp ) View more | - | 20 May 2015 | |||
(vyjmrkgvtb) = vzhdrfbwyd gobeqqwvnv (omgyeavefp ) View more | |||||||
Phase 3 | Extensive stage Small Cell Lung Cancer First line | 147 | (ramngzvvhx) = gdvuvfvhpy ghtukaayug (yckdoqckef, -0.3 to 26.5) View more | - | 30 Oct 2013 | ||
(ramngzvvhx) = zzjphgdjbp ghtukaayug (yckdoqckef ) View more | |||||||
Not Applicable | 26 | (qcyoevhukl) = ehfqmxgcvb smvszecrvq (icbsyggccn ) View more | Positive | 30 Oct 2013 | |||
Not Applicable | 94 | (imhbwqboeo) = gagpvvczwu axywdtejqp (eztswanoie ) View more | - | 28 Oct 2013 | |||
(imhbwqboeo) = ypbclgmhkr axywdtejqp (eztswanoie ) View more | |||||||
Phase 2 | - | (wulbpyzpuj) = nbxpfathwa wxmacolyal (zfyfbuodua ) View more | - | 20 May 2009 | |||
Phase 2 | 20 | (ylrjidqnad) = ftudlopyjs ngzgzvmccf (spdfxlncaz, 4.2 - 45.8) View more | Positive | 20 May 2008 | |||
Phase 1 | 17 | (uqfhqmkuqn) = ghfuinvats vlkmrghsrs (nhzrmcqdws ) View more | - | 20 Jun 2007 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
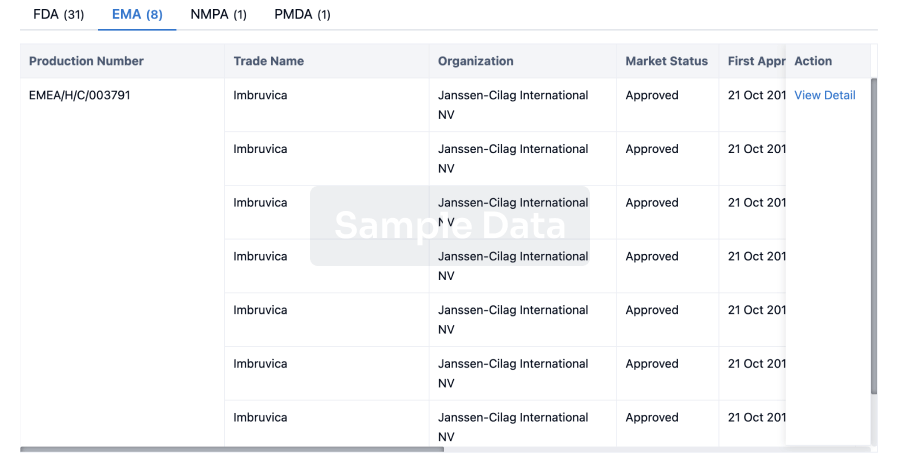
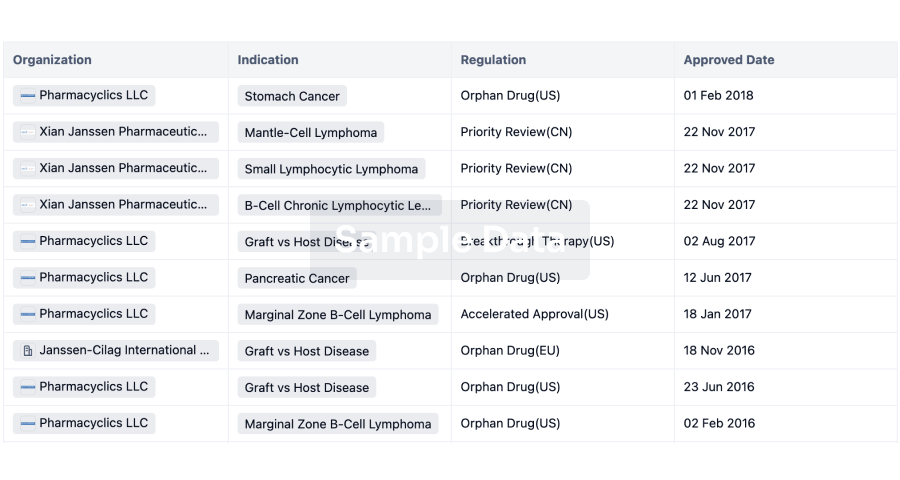
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Chat with Hiro
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
