4DMT Announces Promising Interim Phase 2 PRISM Results for 4D-150 in Varied Wet AMD Patients
4D Molecular Therapeutics, a prominent clinical-stage company specializing in genetic medicines, has revealed promising initial interim 24-week landmark results from the Population Extension cohort of the PRISM Phase 2 Clinical Trial. This study assessed intravitreal 4D-150 in a wide-ranging patient group suffering from wet AMD.
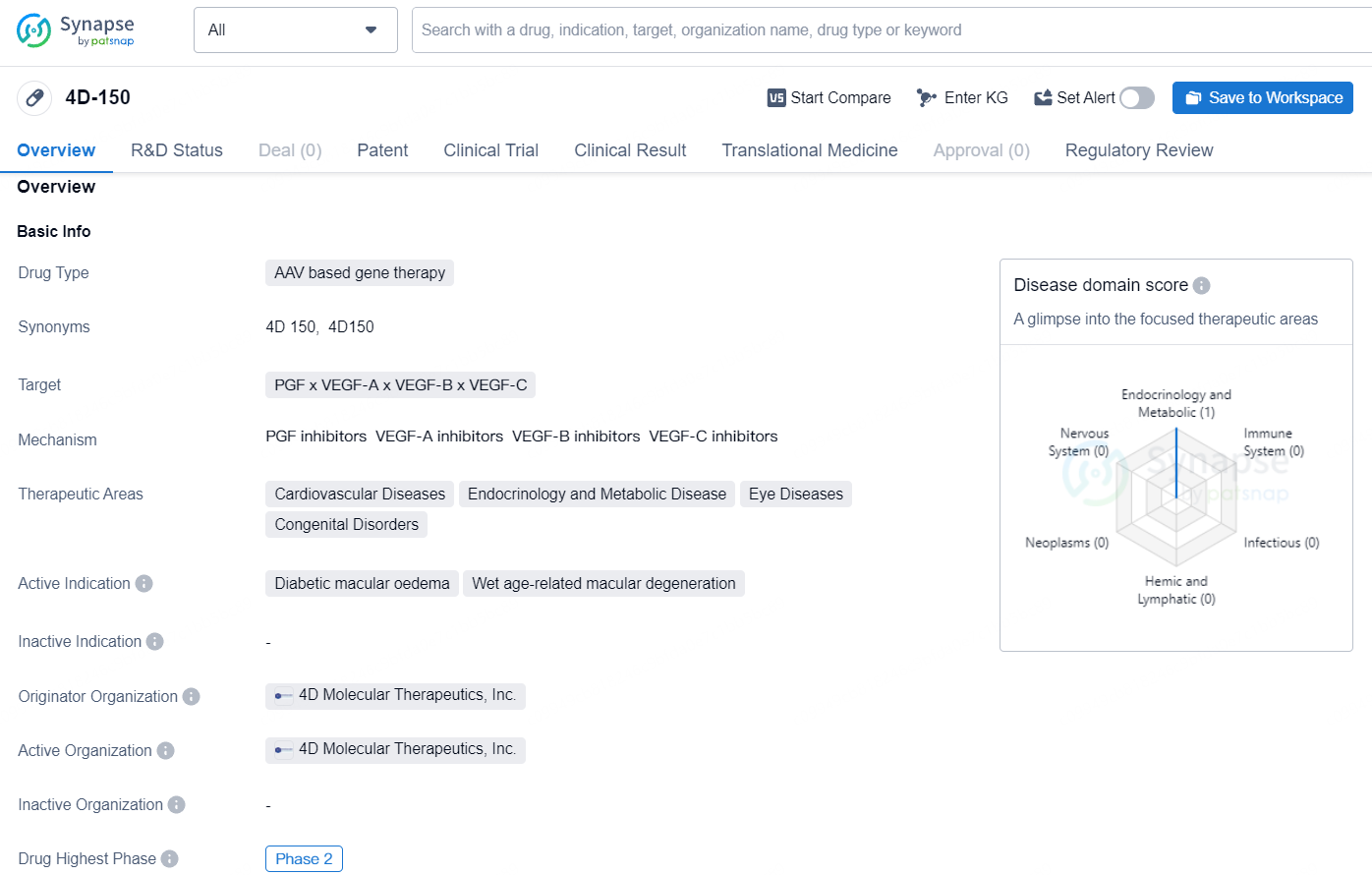
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 The data were shared by Raj K. Maturi, M.D., in an oral presentation titled, "Phase 2 Population Extension Cohort in the PRISM Trial Evaluating 4D-150 in Adults with Neovascular Age-related Macular Degeneration," at the American Society of Retina Specialists Annual Scientific Meeting, which took place in Stockholm, Sweden.
The data were shared by Raj K. Maturi, M.D., in an oral presentation titled, "Phase 2 Population Extension Cohort in the PRISM Trial Evaluating 4D-150 in Adults with Neovascular Age-related Macular Degeneration," at the American Society of Retina Specialists Annual Scientific Meeting, which took place in Stockholm, Sweden.
“The encouraging interim data presented today, along with previously reported results from the Dose Expansion cohort of high treatment burden patients, further validate our planned regulatory strategy and highlight 4D-150’s substantial potential to treat and preserve vision long-term for all patient groups with wet AMD,” said David Kirn, M.D., Co-founder and CEO of 4DMT.
“Moreover, the expanding safety and efficacy database for 4D-150 continues to support the candidate’s potential as a pipeline-in-a-product, offering various multi-billion ophthalmology market opportunities, including wet AMD, DME, and diabetic retinopathy. We expect data readouts from the SPECTRA study in DME in the fourth quarter of this year, which could have implications for DR as well,” added David Kirn.
“The initial benefits of the current repeated bolus anti-VEGF injection treatment paradigm are not sustained long-term in wet AMD patients due to undertreatment and retinal thickness fluctuations, leading to vision deterioration over time,” said Robert Kim, M.D., Chief Medical Officer. “The presented data on 4D-150 continue to demonstrate its promise as a potentially safe, routine, and one-time intravitreal treatment with the long-term goal of preserving vision for millions of wet AMD patients, regardless of their treatment burden or disease severity. We continue to closely collaborate with the FDA and EMA, under our RMAT and PRIME designations, to finalize our Phase 3 clinical trial design, which we anticipate sharing in September 2024.”
“Patients with wet AMD face a significant treatment burden, requiring frequent intravitreal injections throughout their lives, severely impacting quality of life for patients, their families, and caregivers,” said Arshad M. Khanani, M.D., M.A., FASRS, Director of Clinical Research at Sierra Eye Associates and Clinical Professor at the University of Nevada, Reno.
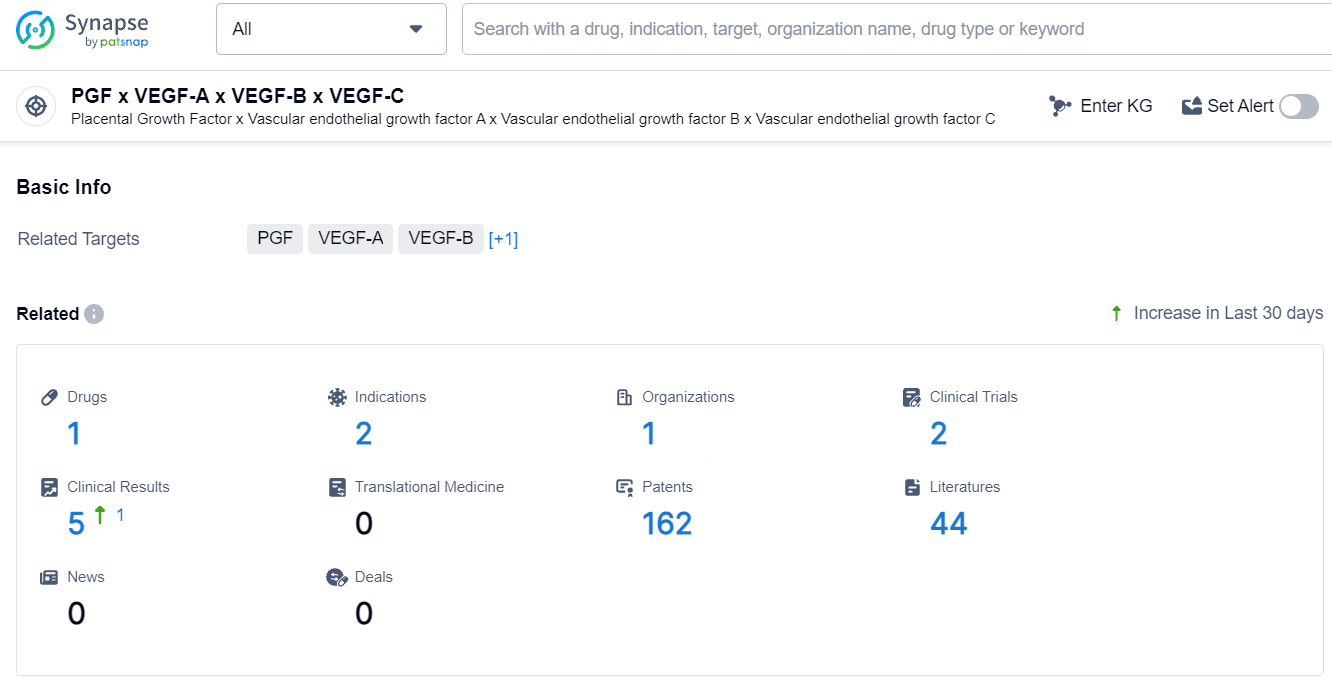
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of July 23, 2024, there are 1 investigational drug for the PGF and VEGF-A and VEGF-B and VEGF-C target, including 2 indications, 1 R&D institution involved, with related clinical trials reaching 2, and as many as 162 patents.
4D-150 is an AAV-based gene therapy drug that targets PGF, VEGF-A, VEGF-B, and VEGF-C, focusing on therapeutic areas such as cardiovascular diseases, endocrinology and metabolic diseases, eye diseases, and congenital disorders. 4D-150 holds promise as an advanced gene therapy drug targeting multiple factors in a range of therapeutic areas, especially cardiovascular and ophthalmic diseases, and its PRIME designation underscores its potential to address significant unmet medical needs.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!