Is Pluvicto approved by the FDA?
Pluvicto, whose generic name is lutetium Lu 177 vipivotide tetraxetan, received FDA approval for its indicated use in adult patients with PSMA-positive mCRPC. Pluvicto This approval allows its use in patients who have previously undergone treatment with other anti-cancer therapies, indicating its efficacy in managing advanced stages of this specific type of prostate cancer.
How Pluvicto Works
Before administering Pluvicto, patients typically undergo imaging using PSMA-11 imaging agents like Locametz to identify PSMA-positive lesions. This step helps ensure targeted treatment delivery to affected areas, optimizing therapeutic outcomes.
Safety Considerations and Warnings
While effective, Pluvicto comes with significant safety precautions due to its nature as a radiopharmaceutical:
- Radiation Exposure: Pluvicto contributes to long-term cumulative radiation exposure, which is managed through strict protocols to minimize risks to patients and caregivers.
- Reproductive Risks: It may cause harm to unborn babies, necessitating effective contraception during treatment and for a specified period afterward.
- Myelosuppression: This condition, marked by reduced blood cell counts, and renal toxicity are serious potential side effects. Patients undergo regular monitoring and may require supportive care to manage these risks effectively.
Administration and Dosage
Pluvicto is administered intravenously every six weeks for a maximum of six doses or until disease progression or unacceptable toxicity occurs. Patients are advised to maintain hydration and follow specific post-administration precautions to minimize radiation exposure to others.
Side Effects
Common side effects of Pluvicto include fatigue, dry mouth, nausea, anemia, decreased appetite, and constipation. Patients should promptly report any signs of myelosuppression or renal toxicity to their healthcare provider.
Drug Interactions
Pluvicto is not known to interact significantly with other medications, though patients should disclose all current medications, including over-the-counter drugs, vitamins, and herbal supplements, to their healthcare provider before treatment initiation.
In conclusion, Pluvicto represents a targeted therapeutic option approved by the FDA for managing PSMA-positive mCRPC in adults who have exhausted other treatment options. Its approval underscores its role in addressing advanced prostate cancer, highlighting ongoing advancements in oncological care.
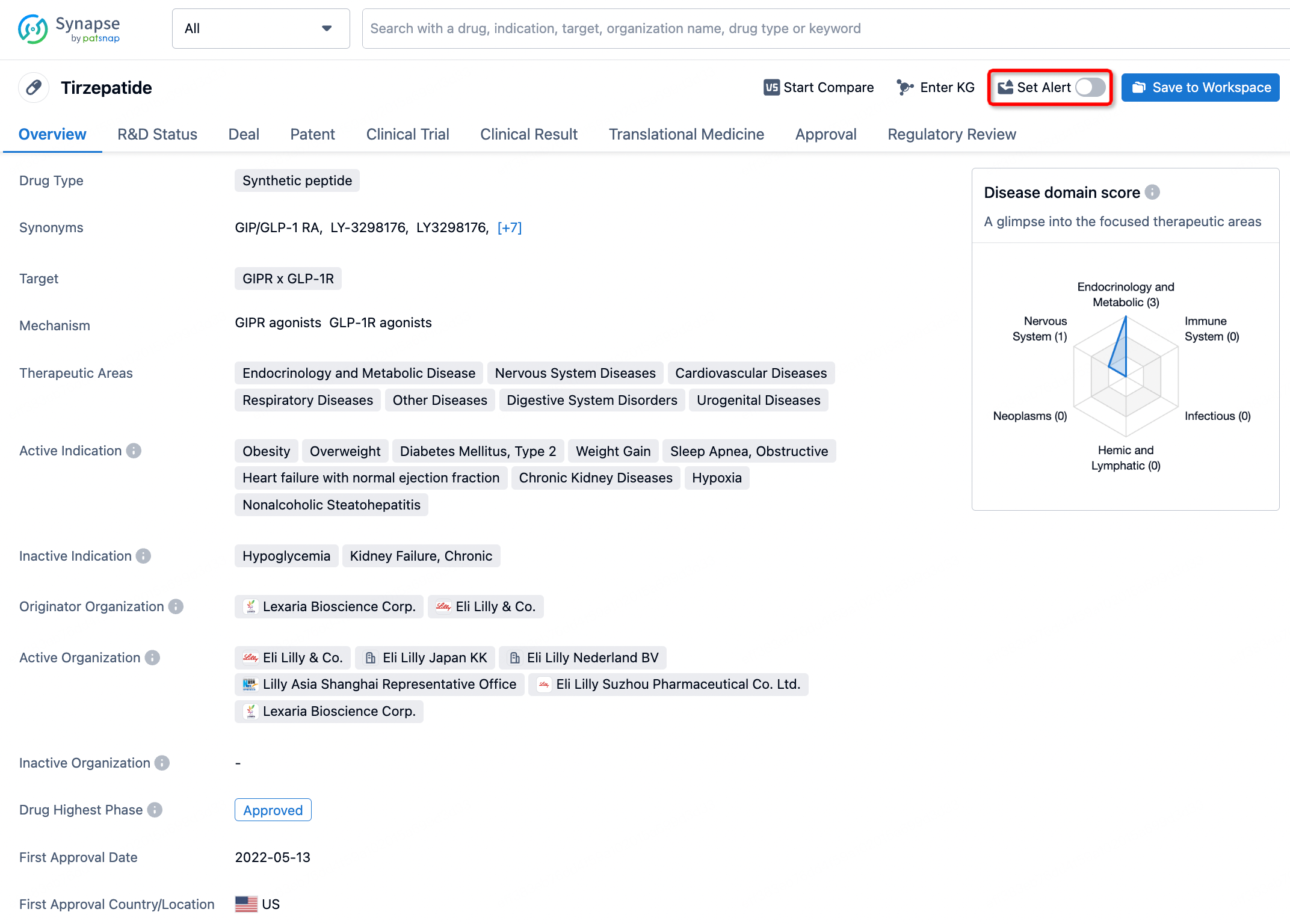
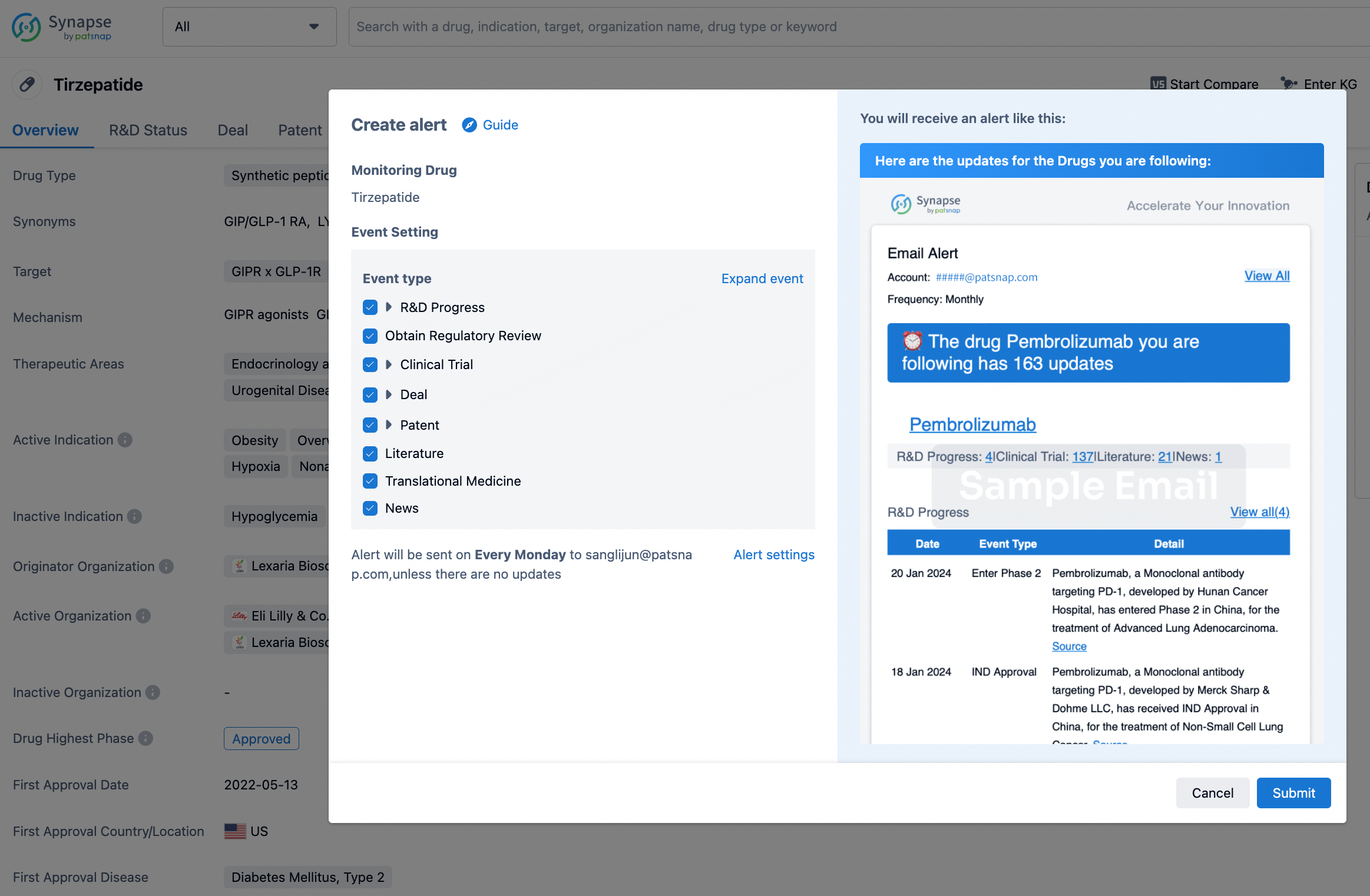
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!