A Phase III trial investigating Novartis' experimental drug, Iptacopan, displayed findings in IgAN patients
Novartis reported encouraging preliminary outcomes from the predefined interim assessment of the Phase III APPLAUSE-IgAN trial over a 9-month period. Iptacopan, an experimental factor B inhibitor focused on the alternate complement pathway, displayed overriding effectiveness compared to the placebo in diminishing proteinuria.
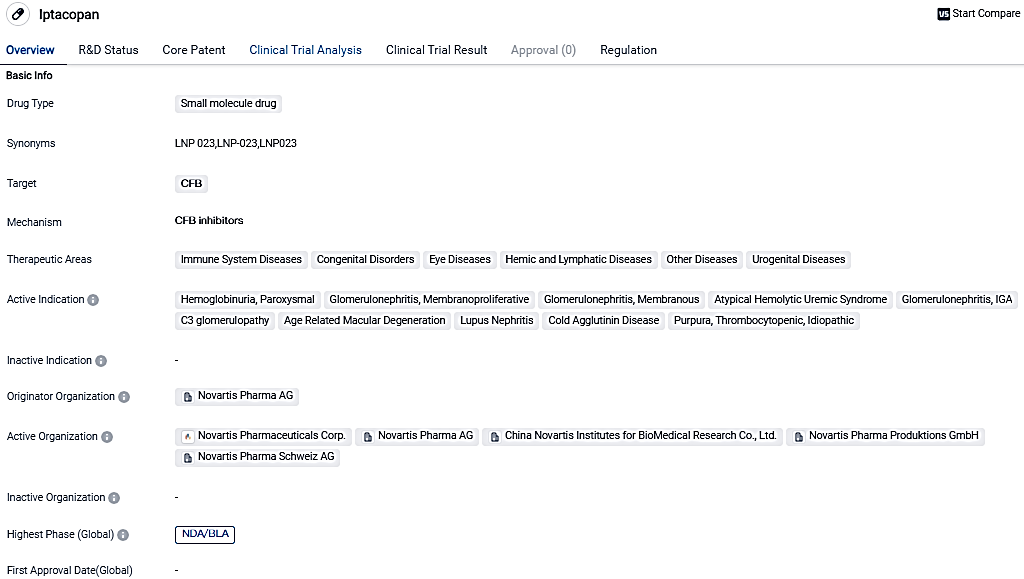
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The study provided a substantial and clinically significant decrease in proteinuria on top of the supportive care for patients battling IgA nephropathy, a disease mediated by the complement system.
Within this research, the safety aspects of iptacopan (200 mg administered twice a day) aligned with prior report findings. The experimental investigation proceeds in an unseen manner, assessing iptacopan's capabilities to impede the advancement of IgAN by monitoring the projected glomerular filtration rate slope for a duration of 24 months. This measurement is the primary focus point by the conclusion of the study, with headline results slated for 2025.
Shreeram Aradhye, M.D., the President of Development and the Chief Medical Officer at Novartis, stated that "The hopeful data from the APPLAUSE study's Phase III highlights the possible positive impact of iptacopan for patients suffering from IgAN, a disabling ailment predominantly afflicting a younger adult demographic. We look forward to the pivotal advancements of our factor B inhibitor of the alternative complement pathway.”
Statistical projection anticipates that every year around 25 individuals per million globally are newly identified with the condition IgAN. A maximum of 30% of those diagnosed with IgAN along with persistently heightened proteinuria levels might experience kidney failure in a 10-year span. Novartis is planning to present a proposal for potential expedited approval to the FDA in 2024.
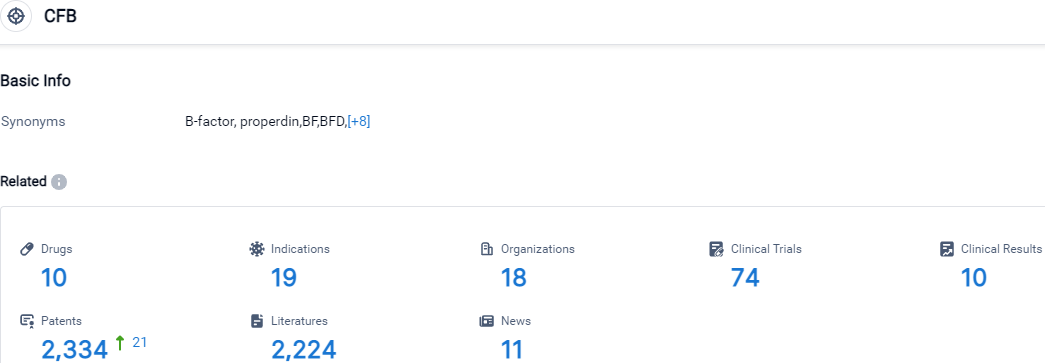
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 9, 2023, there are 10 investigational drugs for the CFB target, including 19 indications, 18 R&D institutions involved, with related clinical trials reaching 74,and as many as 2334 patents.
Iptacopan is an oral, proximal complement inhibitor that binds factor B and inhibits the alternative complement pathway. Based on disease prevalence, unmet needs and data from Phase II studies, iptacopan has received FDA Breakthrough Therapy Designation in PNH.