Abeona Therapeutics Reveals FDA Approval and Priority Review Provision for Pz-cel Biologics License Application (BLA)
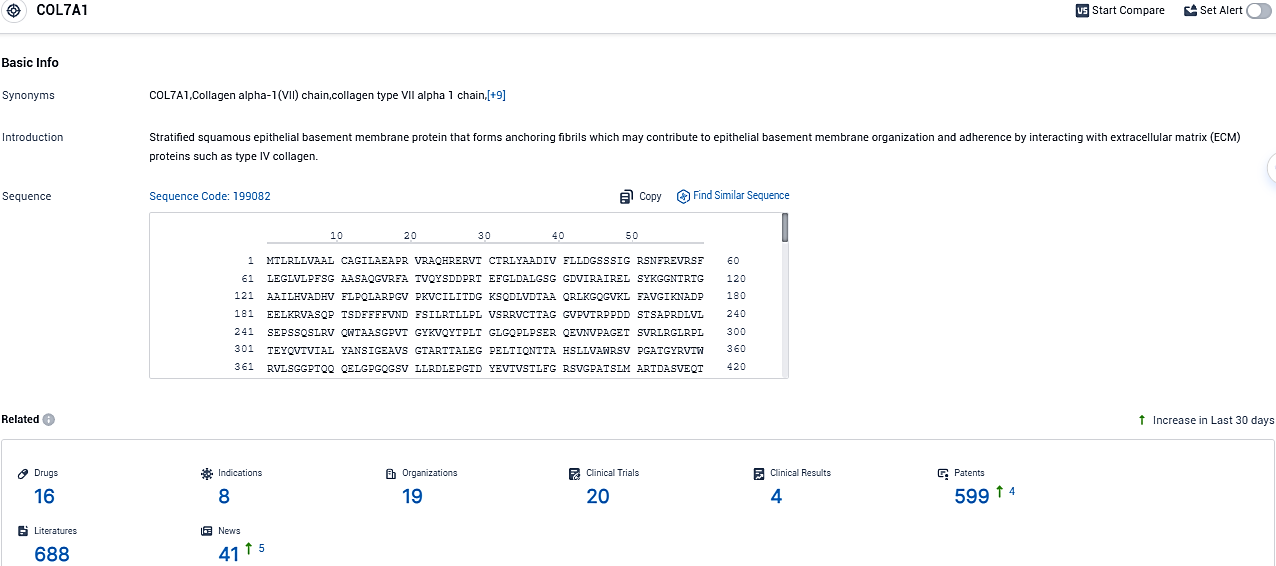
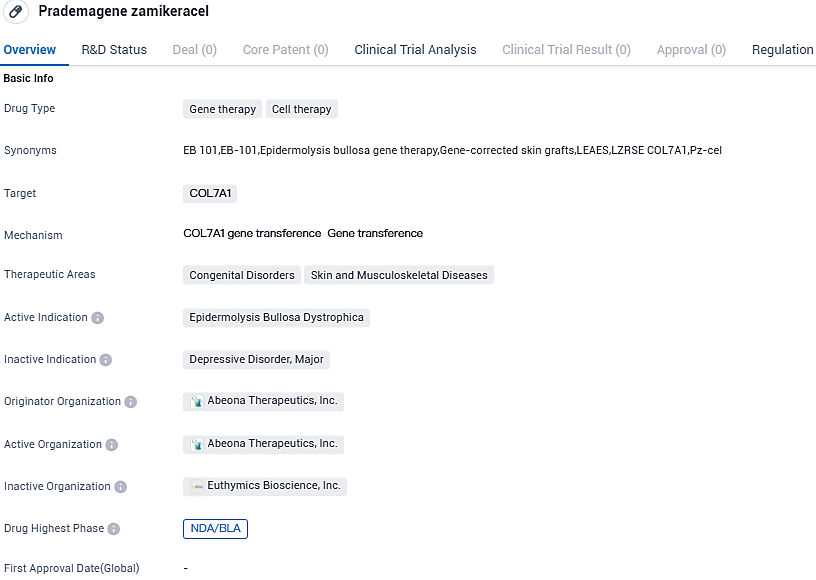
Abeona Therapeutics Inc. has confirmed that the FDA has given priority review status to its Biologics License Application for pz-cel (prademagene zamikeracel), a proprietary therapy developed by the company. This autologous, COL7A1 gene-corrected epidermal sheet technology is specifically devised for treating those affected by recessive dystrophic epidermolysis bullosa.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 Under the legislation of the Prescription Drug User Fee Act, the FDA has aimed to take action by May 25, 2024. Currently, there are no plans by the FDA to organize an Advisory Committee discussion for the pz-cel application.
Under the legislation of the Prescription Drug User Fee Act, the FDA has aimed to take action by May 25, 2024. Currently, there are no plans by the FDA to organize an Advisory Committee discussion for the pz-cel application.
“Receiving the BLA priority review from the FDA emphasizes the severe lack of solutions for RDEB, and signifies the potential of pz-cel to make a significant difference for these patients,” commented Vish Seshadri, CEO of Abeona. “We are grateful to the FDA for their dedication and are eager to collaborate with them throughout the BLA assessment, aiming to bring this treatment to patients as rapidly as possible.”
The BLA is reinforced by clinical efficacy and safety outcomes from the crucial Phase 3 VIITAL™ study, as well as confirmatory evidence from a Phase 1/2a study. Both studies demonstrated that a single use of pz-cel on large and chronic wounds facilitated long-lasting wound healing and alleviated pain.
The acknowledgment of the Priority Review status is a significant initial step for Abeona’s possibility of obtaining a Priority Review Voucher. Pz-cel (prademagene zamikeracel), an experimental autologous, COL7A1 gene-corrected epidermal sheets from Abeona, is under development at present for treating recessive dystrophic epidermolysis bullosa, a unique connective tissue disorder resulting from a defect in the COL7A1 gene which inhibits the production of Type VII collagen.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 30, 2023, there are 16 investigational drugs for the COL7A1 target, including 8 indications, 19 R&D institutions involved, with related clinical trials reaching 20, and as many as 599 patents.
The development of Prademagene zamikeracel represents a significant advancement in the field of biomedicine, particularly in the treatment of rare genetic disorders. If approved, this gene and cell therapy drug has the potential to provide a much-needed therapeutic option for patients with Epidermolysis Bullosa Dystrophica, improving their quality of life and potentially reducing the burden of long-term complications associated with the disease.