ABL Bio to Collaborate on Testing ABL103 with KEYTRUDA® (pembrolizumab) for Solid Tumor Patients
ABL Bio, led by CEO Sang Hoon Lee and known for its work on bispecific antibodies, revealed that it has formed a clinical trial collaboration and supply agreement with MSD, a branch of Merck & Co., Inc. located in Rahway, NJ, USA. This partnership aims to assess the effectiveness of combining ABL103 with MSD’s anti-PD-1 treatment, KEYTRUDA® (pembrolizumab), in individuals suffering from advanced or metastatic solid tumors.
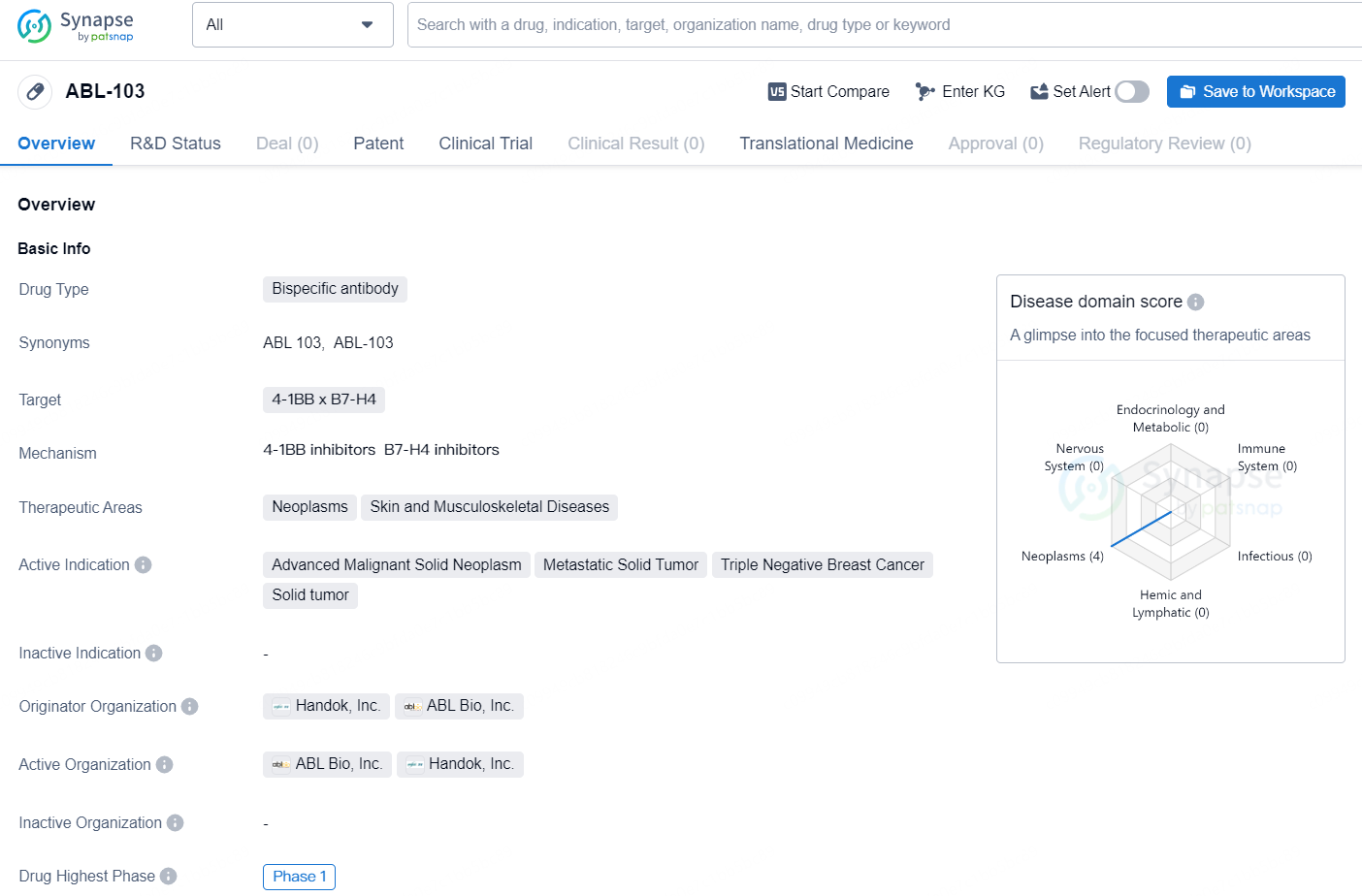
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
According to the agreement, ABL Bio is set to carry out a phase 1b/2 clinical trial to assess the safety and effectiveness of combining ABL103 with KEYTRUDA. In this joint study, MSD will provide KEYTRUDA. ABL103 is a bispecific antibody targeting both B7-H4 and 4-1BB. It is part of ABL Bio's pipeline programs that utilize the Grabody-T platform, a 4-1BB based bispecific antibody technology. Grabody-T is engineered to activate T cells exclusively within the tumor microenvironment, reducing liver toxicity observed with traditional 4-1BB monoclonal antibodies while boosting anticancer effectiveness.
The mechanism of ABL103 involves activating the 4-1BB signaling pathways within tumor microenvironments harboring B7-H4 antigens. This allows T cells to target tumor cells selectively, minimizing damage to healthy cells. Presently, the phase 1 clinical trial’s dose escalation phase for ABL103 is taking place in South Korea.
The CEO of ABL Bio, Sang Hoon Lee, stated, "We are happy to initiate this clinical collaboration with MSD. With this deal, we are prepared to progress with the clinical development of ABL103. We anticipate that combining ABL103 with KEYTRUDA will enhance the lives of patients with advanced or metastatic solid tumors. So far, the phase 1 trial for ABL103 as a monotherapy has been proceeding well, and we are committed to achieving significant outcomes from ABL103 through clinical development."
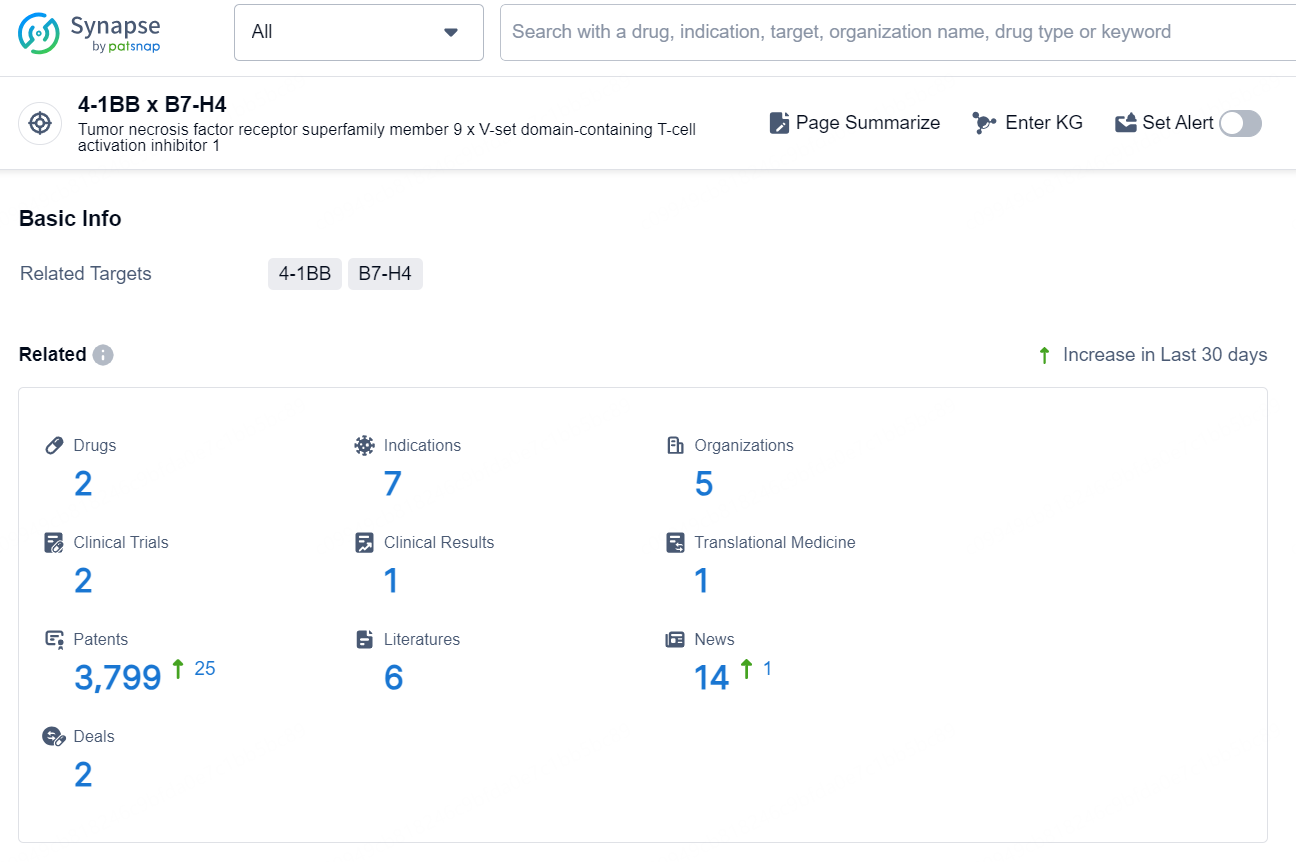
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 9, 2024, there are 2 investigational drug for the 4-1BB x B7-H4 targets, including 7 indications, 5 R&D institution involved, with related clinical trials reaching 2, and as many as 3799 patents.
ABL-103 is a bispecific antibody drug currently in development, with a focus on targeting the 4-1BB x B7-H4 pathways in the treatment of neoplasms, skin, and musculoskeletal diseases. The drug is intended for use in the treatment of advanced malignant solid neoplasms, metastatic solid tumors, triple-negative breast cancer, and solid tumors.