Acadia Pharmaceuticals starts the Phase 2 study of ACP-204 in treating Alzheimer's Disease-related psychosis
Acadia Pharmaceuticals Inc. has declared the commencement of a Phase 2 trial to assess the effectiveness and safety of ACP-204 in treating hallucinations and delusions linked to psychosis caused by Alzheimer's disease. Out of over 6.5 million Alzheimer's patients in the United States, around 30% are likely to undergo psychosis, usually characterized by hallucinations and delusions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
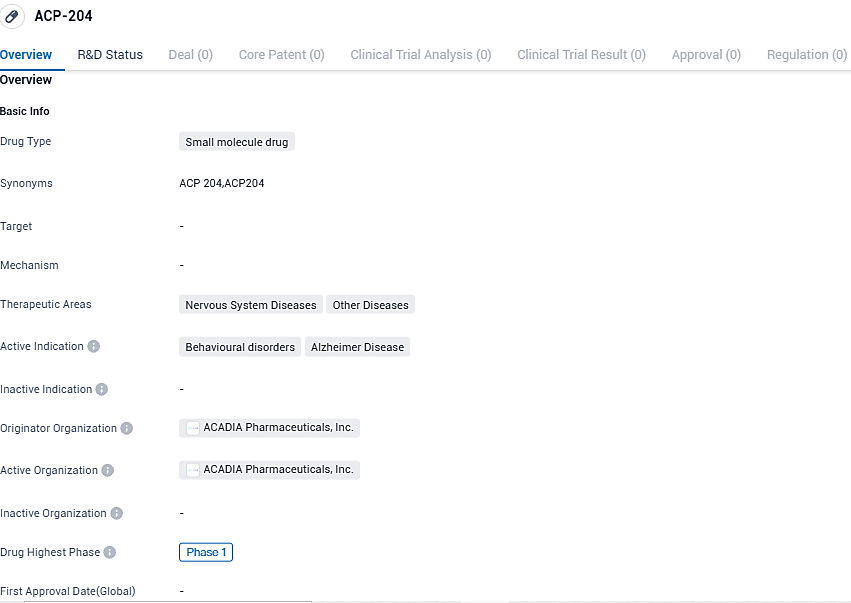
The second phase trial forms a component of an integrated program for Phase 2 and Phase 3, comprising three separate studies. These include a solitary Phase 2 study and two analogous Phase 3 studies. The second phase of this international, multi-site, randomized, double-blind, placebo-controlled study aims to recruit roughly 318 participants to analyze the effects of ACP-204 dosages of 30mg and 60mg in comparison to a placebo.
The primary objective here signifies a change from the base level in the grading system for evaluating positive symptoms, specifically hallucinations as well as the extent of delusions, all of which are examined with the final score after a six-week duration. It is planned that the clinical research locations will transition smoothly from Phase 2 to Phase 3. Each upcoming Phase 3 study intends to enroll around 378 patients diagnosed with ADP. After completing the study, participants are given the choice to partake in an open-label extension study for an extended period.
As per the Alzheimer’s Association, Alzheimer's disease affects more than 6.5 million individuals in the U.S. It's estimated that about 30% of Alzheimer's patients experience psychosis, often characterized by hallucinations and delusions. These symptoms can be persistent, severe, and may resurface intermittently. Hallucinations are identified as seeming perception-like experiences which occur absent any external stimulus and typically have a sensory aspect. Currently, the FDA has yet to approve a specific drug for Alzheimer's disease psychosis.
ACP-204 primarily functions as an inverse agonist targeting the 5-HT2A receptor. Currently, this compound is undergoing development as a potential treatment for Alzheimer's psychosis, a condition with no FDA-approved treatment, representing a significant medical need.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
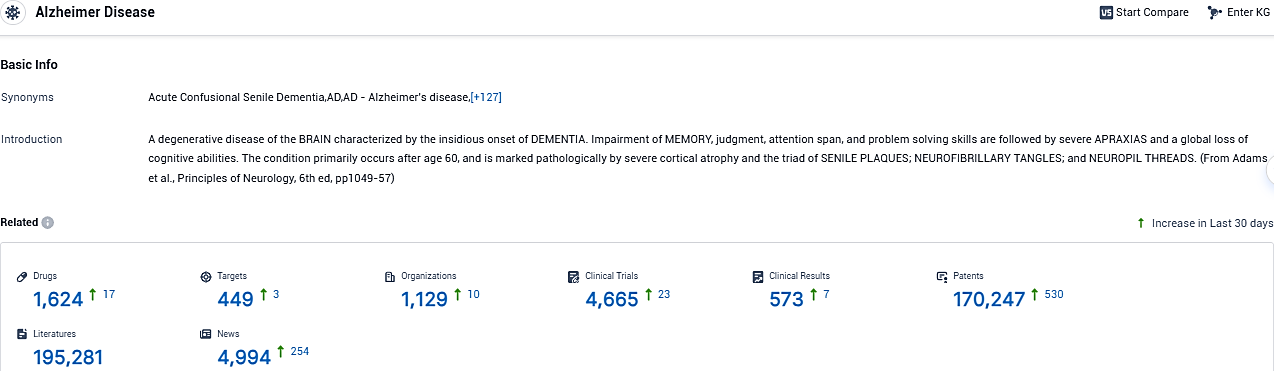
According to the data provided by the Synapse Database, As of November 30, 2023, there are 1624 investigational drugs for Alzheimer Disease, including 449 targets, 1129 R&D institutions involved, with related clinical trials reaching 4665, and as many as 170247 patents.
ACP-204 is being investigated for its potential therapeutic effects in the areas of Nervous System Diseases and Other Diseases, specifically targeting Behavioural disorders and Alzheimer's Disease. As it is currently in Phase 2 of clinical development, further studies are needed to evaluate its safety and efficacy in larger patient populations.