Deciphering IL-6 Inhibitors: Your Guide to Rapidly Accessing the Newest Advances
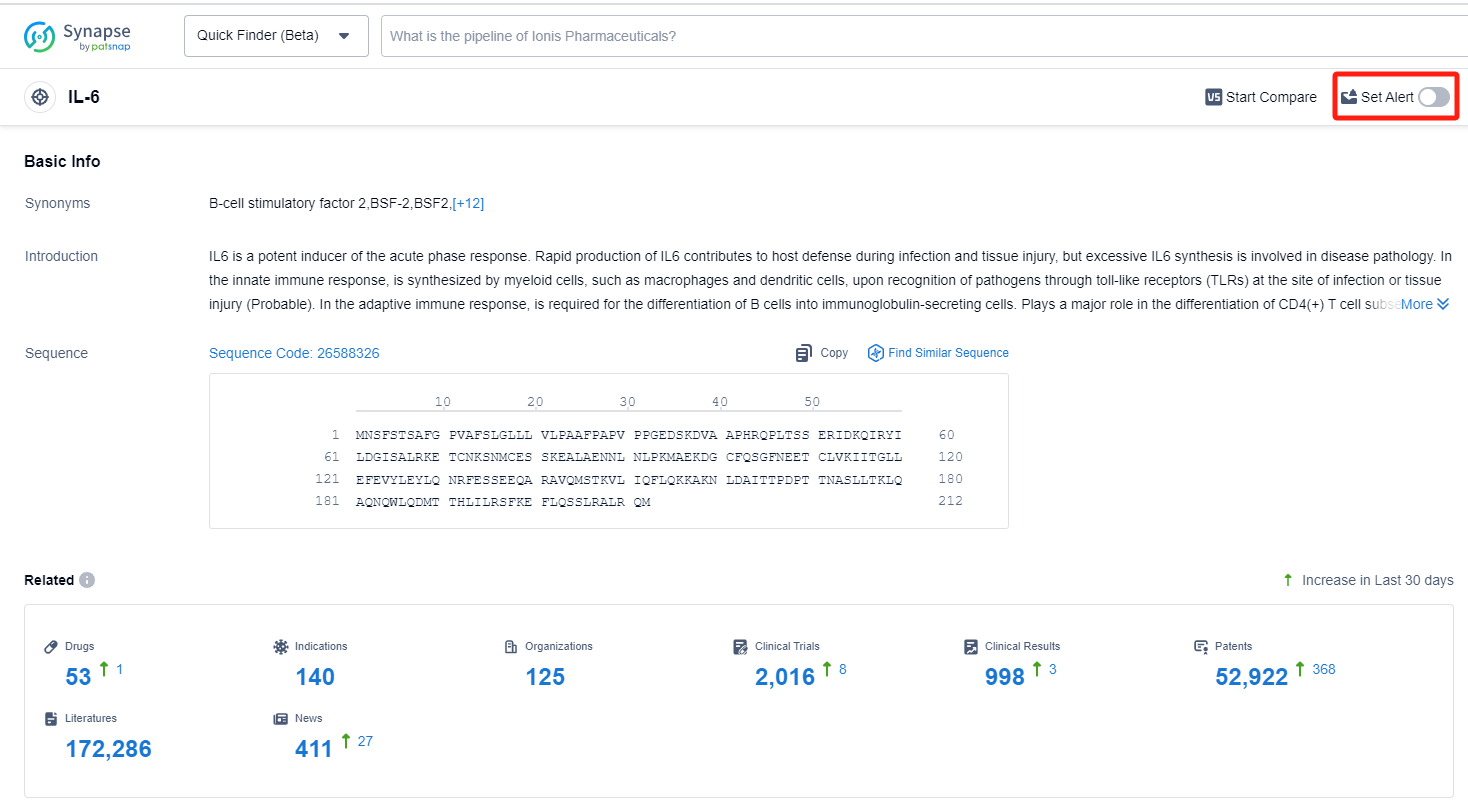
IL-6, or Interleukin-6, is a cytokine that plays a crucial role in the human body's immune response and inflammation regulation. It is produced by various cells, including immune cells, and acts as a signaling molecule to stimulate the immune system during infection or injury. IL-6 also plays a role in the development and differentiation of immune cells, such as B cells and T cells. However, excessive or prolonged IL-6 production can lead to chronic inflammation and contribute to the pathogenesis of various diseases, including autoimmune disorders and certain cancers. Therefore, understanding and regulating IL-6 levels is of great importance in the pharmaceutical industry for the development of targeted therapies.
IL-6 Inhibitors is relevant to many inflammatory diseases and cancers. The first approved medication in this class was tocilizumab (Actemra), an antibody directed against the IL-6 receptor. This was followed by siltuximab (Sylvant), which is directed against IL-6 itself. Sarilumab was approved by the US FDA in 2017 for rheumatoid arthritis. Several other agents are in clinical trials, including olokizumab (CDP6038), elsilimomab, clazakizumab (BMS-945429, ALD518), sirukumab (CNTO 136), levilimab (BCD-089), and CPSI-2364.
Looking ahead, IL-6 inhibitors are expected to continue to play a significant role in the treatment of various diseases. They have shown promising results in treating serious COVID-19 cases2 and are also used widely for the treatment of rheumatoid arthritis2. The IL-6 inhibitors market is projected to grow at a CAGR of 10.9%, increasing from US$ 33.3 Billion in 2023 to US$ 93.3 Billion by 20333. However, the emergence of resistance and the lack of an effective reversal drug when bleeding occurs are challenges that need to be addressed. Ongoing research and development efforts are focused on addressing these issues and expanding the clinical applications of IL-6 inhibitors.
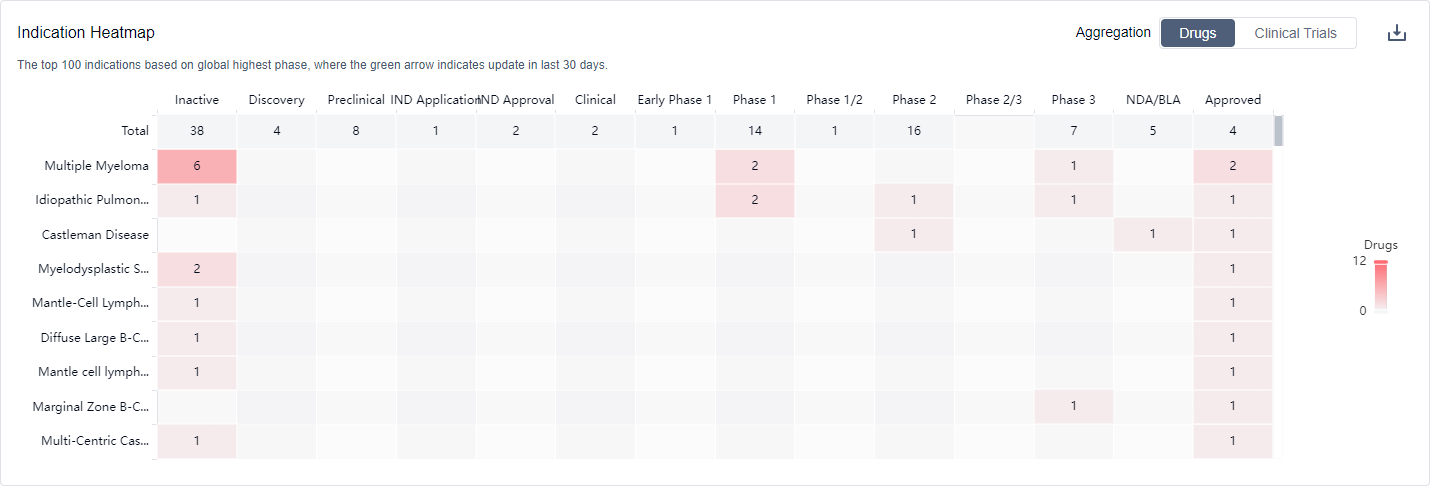
Based on the analysis of the data, the current competitive landscape for target IL-6 is characterized by the presence of multiple companies with varying stages of development and a wide range of indications. Bristol Myers Squibb Co. stands out as the company with the highest stage of development, with multiple approved drugs and ongoing clinical trials. Small molecule drugs, molecular glues, and monoclonal antibodies are the most rapidly progressing drug types under the target IL-6. The United States, European Union, and China are the leading countries/locations in terms of development, with China showing significant progress. Overall, the target IL-6 presents a competitive landscape with diverse players and promising future development potential.
How do they work?
IL-6 inhibitors are a type of medication that target and inhibit the activity of interleukin-6 (IL-6), a cytokine involved in the immune response and inflammation. From a biomedical perspective, IL-6 inhibitors are used in the treatment of various inflammatory conditions such as rheumatoid arthritis, systemic juvenile idiopathic arthritis, and Castleman's disease. These inhibitors work by blocking the binding of IL-6 to its receptor, thereby reducing the inflammatory response mediated by IL-6. By inhibiting IL-6, these medications help alleviate symptoms and reduce disease progression in patients with IL-6-mediated inflammatory disorders
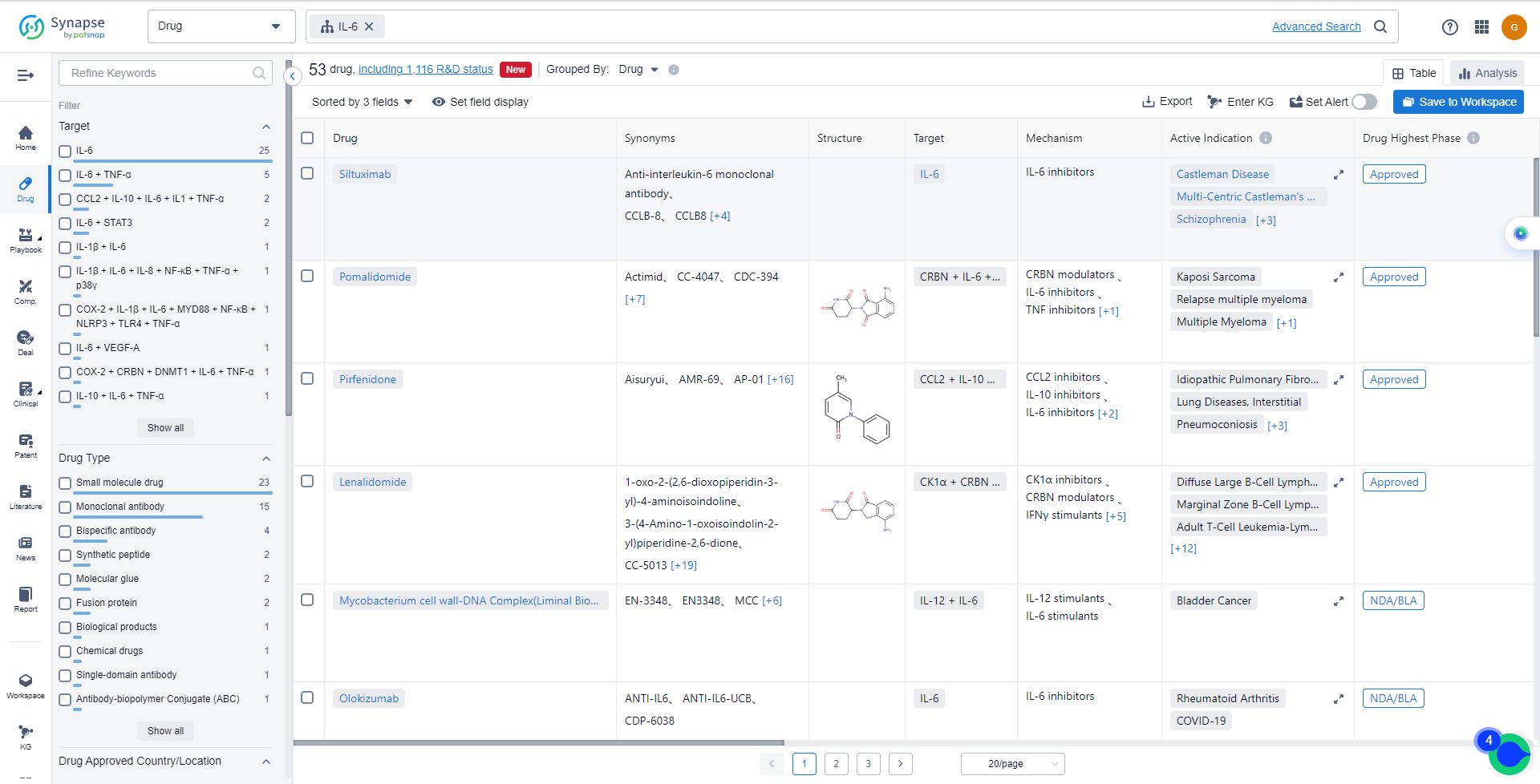
List of IL-6 Inhibitors
The currently marketed IL-6 inhibitors include:
- Siltuximab
- Pomalidomide
- Pirfenidone
- Lenalidomide
- Olokizumab
- Sirukumab
- Sodium Pyruvate
- Clazakizumab
- RO-7200220
- Ziltivekimab
For more information, please click on the image below.
What are IL-6 inhibitors used for?
IL-6 inhibitors are used in the treatment of various inflammatory conditions such as rheumatoid arthritis, systemic juvenile idiopathic arthritis, and Castleman's disease. For more information, please click on the image below to log in and search.
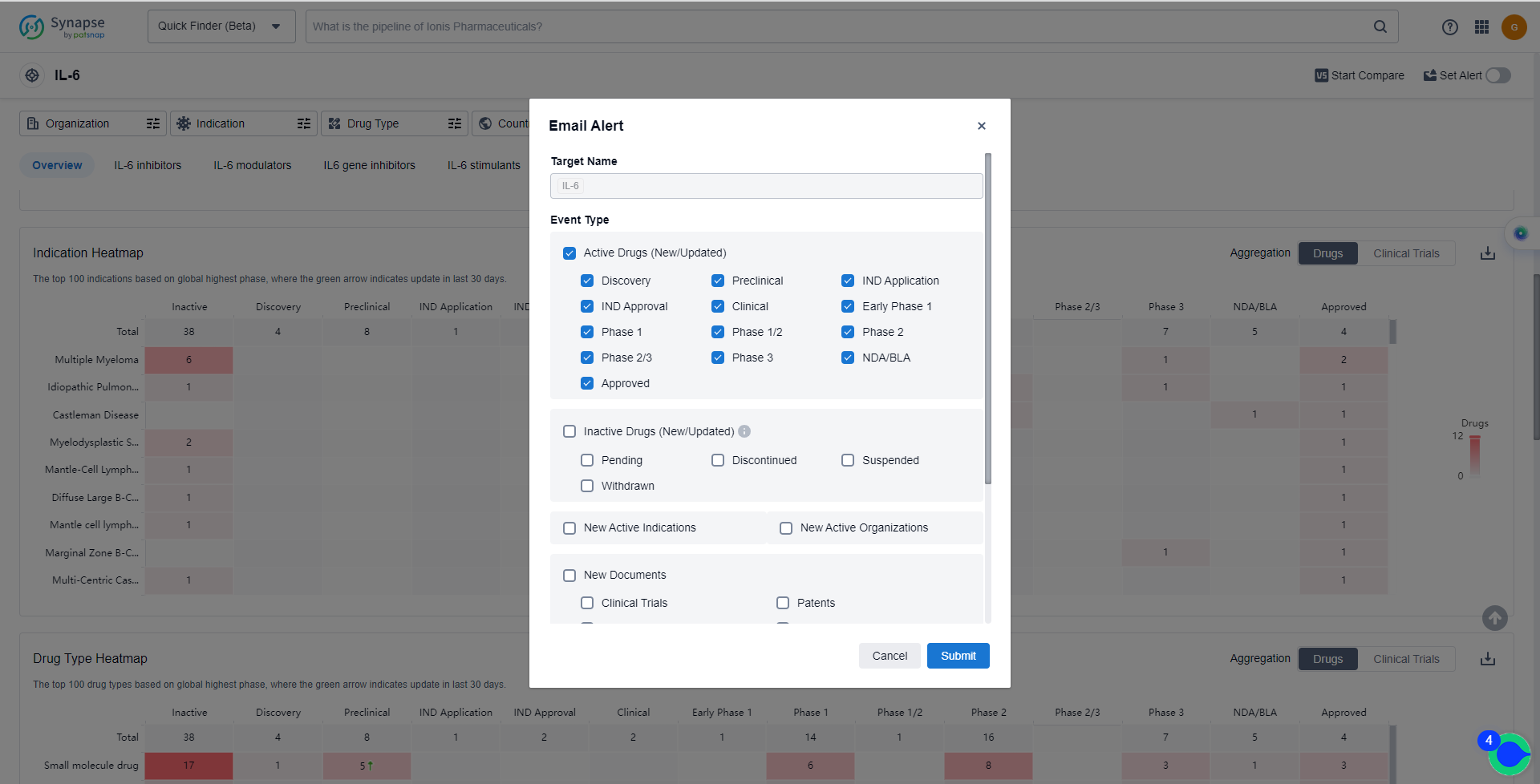
How to obtain the latest development progress of IL-6 inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of IL-6 inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!