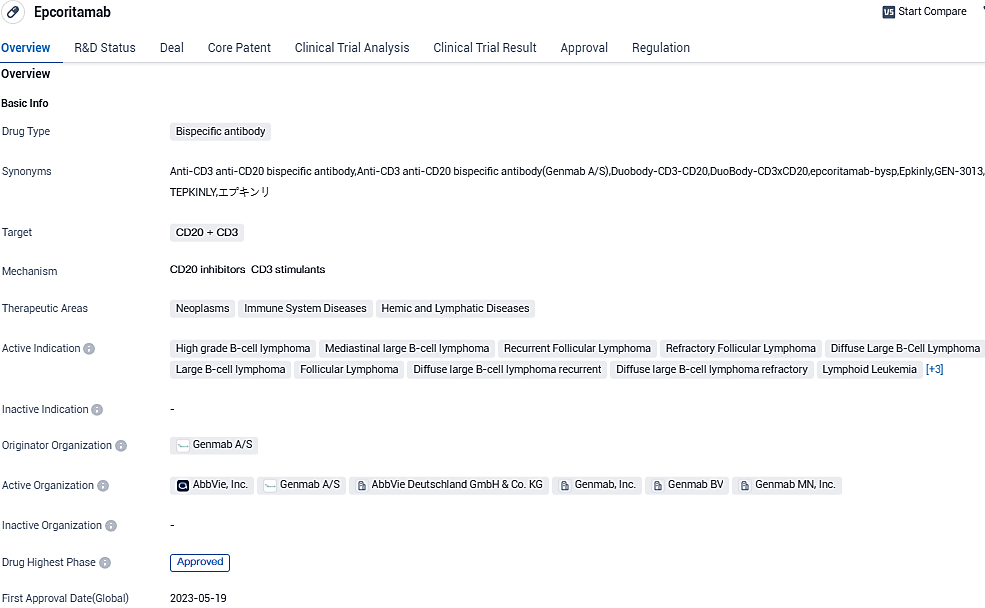
Genmab Reports Encouraging Regulatory Progress for Epcoritamab (EPKINLY®/TEPKINLY®) in Treating Recurring/Resistant Follicular Lymphoma
Genmab A/S shared updates on regulatory matters from the US Food and Drug Administration and European Medicines Agency regarding epcoritamab, a subcutaneously administered bispecific antibody that engages T-cells, currently under investigation.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The U.S. FDA has awarded the Breakthrough Therapy Designation (BTD) for epcoritamab-bysp's use in treating adult patients facing a relapsed or refractory follicular lymphoma post two or more systemic therapy courses. The purpose of BTD is to ensure more rapid progress and examination of medicines under trial by the FDA for severe or potentially fatal diseases where initial clinical proof suggests significant potential advancements over existing treatments.
Furthermore, the EMA has approved a Type II variation application for prescribing epcoritamab for an equivalent health condition. EMA approval ensures the application is comprehensive and initiates the scientific evaluation procedure by the EMA’s Committee for Medicinal Products for Human Use. If consented, R/R FL could be the additional conditionally endorsed application for epcoritamab inside the European Union.
"There is a continuing demand for more treatment strategies for relapsed or refractory follicular lymphoma, despite the amelioration in treatments," articulated Jan van de Winkel, Ph.D., Genmab’s Chief Executive Officer. "The positive responses from the regulatory bodies are reassuring, and the likelihood is high that these may expedite the provision of epcoritamab to those suffering from this illness."
The regulatory decisions received endorsement from the results previously disclosed from the EPCORE NHL-1 clinical phase 1/2 trial, a multi-center, open-label study determining the safety and initial effectiveness of subcutaneous epcoritamab in 128 adult patients with relapsed, refractory CD20+ mature B-cell non-Hodgkin’s lymphoma inclusive of FL.
Composed utilizing Genmab's proprietary DuoBody® technology, Epcoritamab is an IgG1-bispecific antibody issued subcutaneously. The purpose of Genmab's DuoBody- CD3 technology is to guide cytotoxic T cells selectively to generate an immune response against specific cell types. Epcoritamab is devised to concurrently bind to CD3 on T cells and CD20 on B cells and initiate T-cell-mediated extermination of CD20+ cells.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
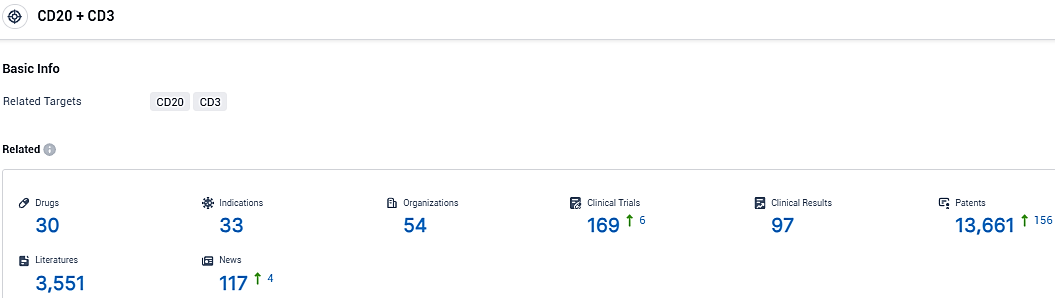
According to the data provided by the Synapse Database, As of November 30, 2023, there are 30 investigational drugs for the CD20 and CD3 target, including 33 indications, 54 R&D institutions involved, with related clinical trials reaching 169, and as many as 13661 patents.
Epcoritamab approved under the brand name EPKINLY in the U.S. and Japan, and TEPKINLY in the EU.It has received regulatory approval in certain lymphoma indications in several territories. Use of epcoritamab in FL is not approved in the U.S. or in the EU. Epcoritamab is being co-developed by Genmab and AbbVie as part of the companies' oncology collaboration. The companies will share commercial responsibilities in the U.S. and Japan, with AbbVie responsible for further global commercialization.