Accent Therapeutics Begins ATX-559 Trial and Appoints New CSO
Accent Therapeutics, a biopharmaceutical firm in the clinical stage focused on innovative, targeted small molecule therapies for cancer, has announced that the initial patient has received a dose in the Phase 1/2 clinical trial assessing the safety and tolerability of ATX-559, a pioneering oral inhibitor of DHX9. Additionally, the company reported the retirement of Robert A. Copeland, Ph.D., who served as Co-Founder, President, and Chief Scientific Officer (CSO), while promoting Serena Silver, Ph.D., to the role of CSO.
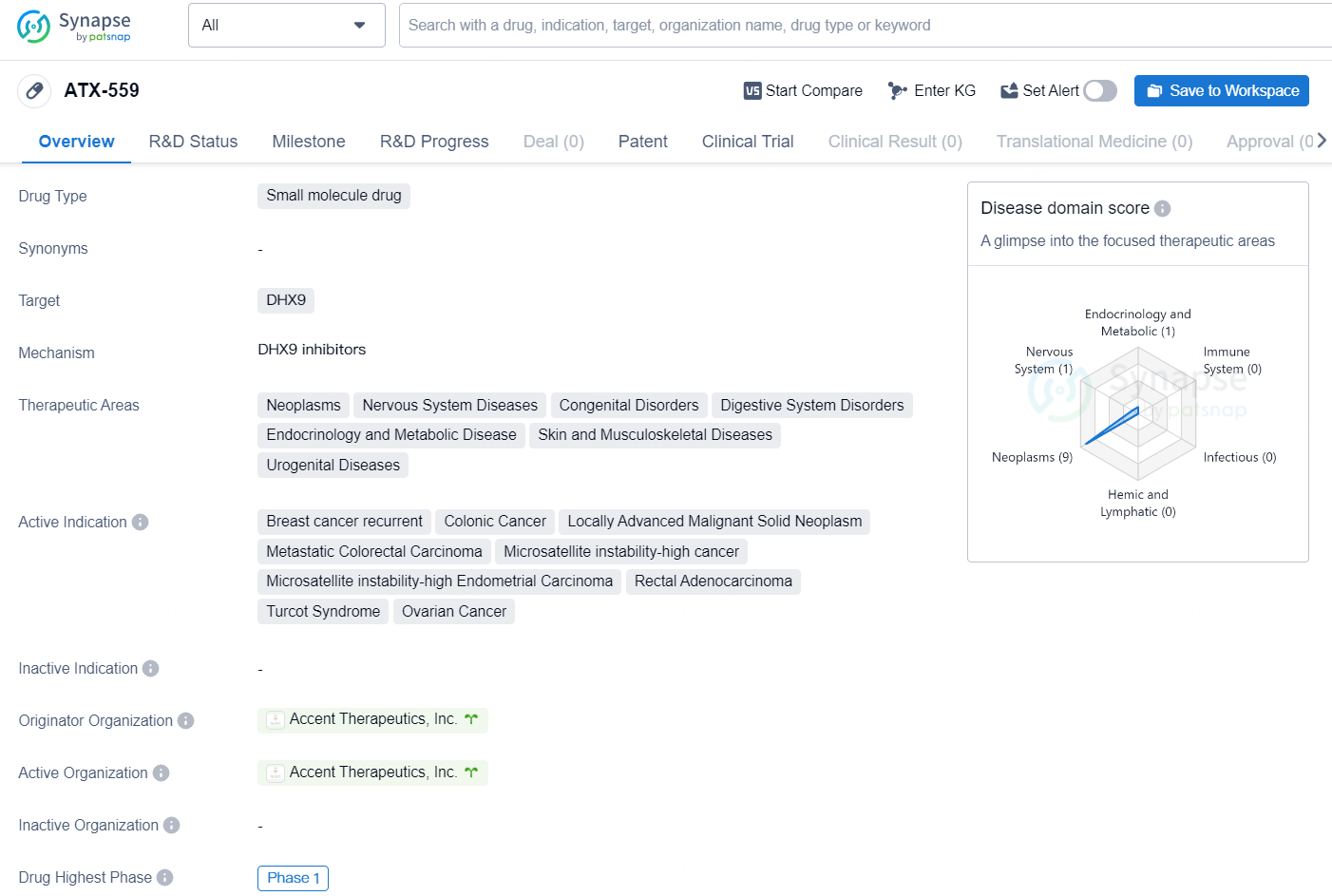
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
ATX-559 is a pioneering and highly selective inhibitor of DHX9, an unprecedented RNA and DNA/RNA helicase that has not been targeted by any existing drugs. Studies have indicated that DHX9 is essential in cancers exhibiting significant replication stress, including breast, ovarian, colorectal, endometrial, and gastric cancers, highlighting a substantial patient population with considerable unmet medical requirements. The ongoing Phase 1/2 trial of ATX-559 (NCT06625515) aims to assess its safety across various dosage levels while evaluating tolerability, pharmacokinetics, pharmacodynamics, and initial efficacy. This trial is actively recruiting patients with solid tumors, particularly those with BRCA1 or BRCA2 deficiencies, as well as individuals with MSI-H and/or dMMR solid tumors (which encompass certain colorectal, endometrial, gastric, and other malignancies). The progression of ATX-559 into the clinical phase, followed by the KIF18A initiative expected to enter trials in the first half of 2025, signifies a vital achievement for the company in developing potentially transformative cancer treatments.
"We are excited to commence the evaluation of ATX-559 in patients with cancer. Although PARP inhibitors serve as standard therapies for breast cancer patients lacking BRCA1 or BRCA2, most individuals with metastatic disease will likely require alternative treatment within one or two years. Similarly, nearly half of the patients with MSI-H/dMMR colorectal cancer may need additional therapies after receiving PD-(L)1 inhibitors," stated Jason Sager, M.D., Chief Medical Officer at Accent Therapeutics. "With ATX-559 advancing into clinical trials and our KIF18A inhibitor ready to launch Phase 1 trials, we are strategically positioned to convert our comprehensive scientific research into the creation of several new treatments for cancer. These initiatives reflect years of pioneering research and scientific rigor from the Accent Therapeutics team as we strive to offer meaningful new therapeutic possibilities for patients facing these difficult cancers."
Effective from January 1, 2025, Serena Silver, Ph.D., will ascend to the role of Chief Scientific Officer. Dr. Silver joined Accent in September 2022, serving as Vice President of Biology, and brought extensive experience in target discovery, drug development, and translational research. Prior to her tenure at Accent, she was Vice President of Discovery Biology and Technologies at Fulcrum Therapeutics, where she directed teams to create and implement new assay techniques and complex cellular models for disease to identify therapeutic targets for rare conditions. Additionally, Dr. Silver has led the Molecular Pharmacology group at Novartis Oncology and the Target ID and Validation team at Sanofi Oncology, and she worked at the cutting-edge of functional genomics at the Broad Institute. She earned her Ph.D. in Biology from the Massachusetts Institute of Technology and completed a postdoctoral fellowship at Harvard Medical School.
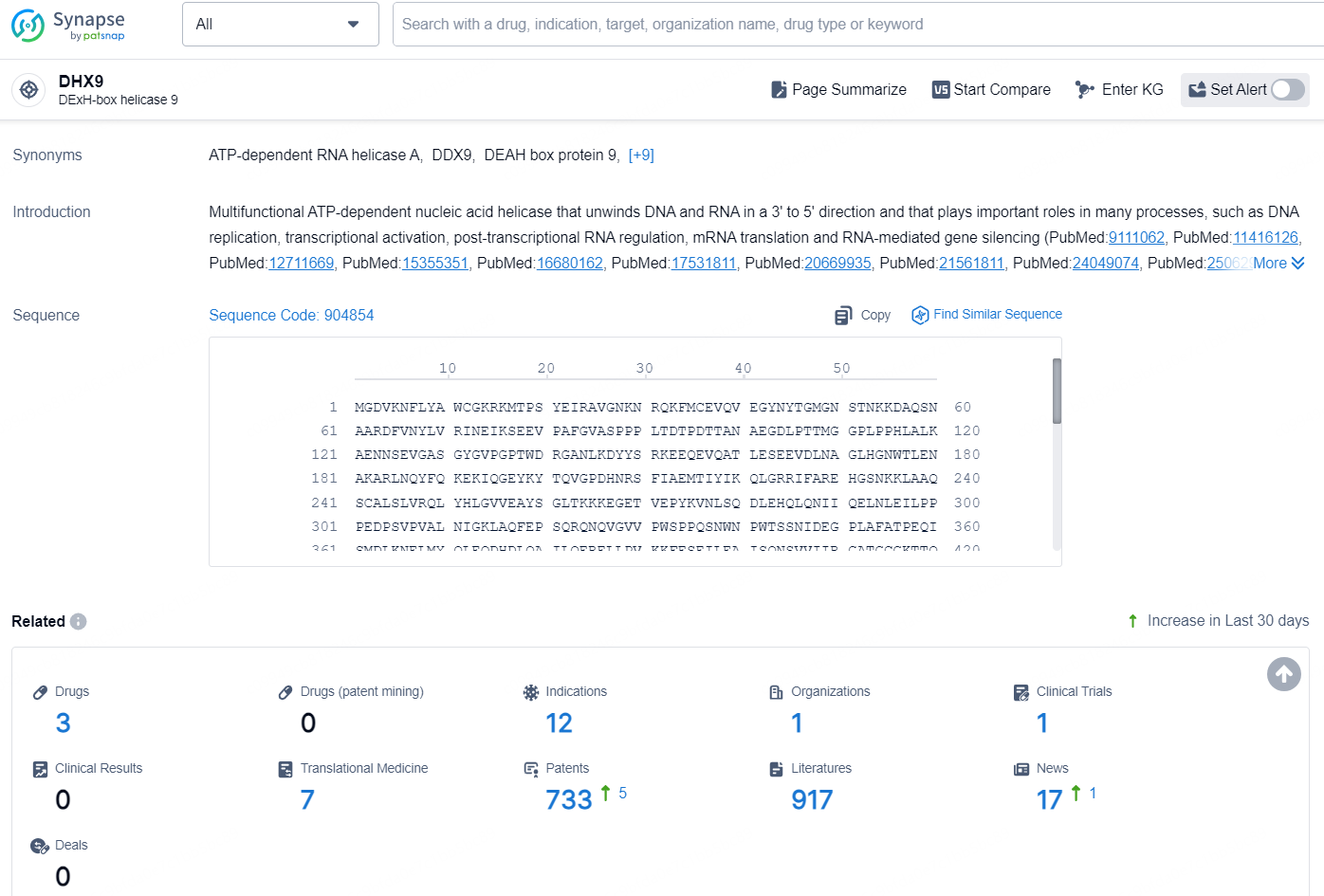
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of December 18, 2024, there are 3 investigational drugs for the DHX9 target, including 12 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 733 patents.
ATX-559 is a small molecule drug developed by Accent Therapeutics, Inc., with a primary target of DHX9. The drug is currently in Phase 1, indicating that it is in the early stages of clinical development. The therapeutic areas that ATX-559 aims to address include neoplasms, nervous system diseases, congenital disorders, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases. This suggests that the drug has potential applications in a wide range of medical conditions.