ACM Biolabs Releases Encouraging Findings from a Phase I Study for SARS-CoV-2 Enhancer Vaccine ACM-001
ACM Biolabs, a firm specializing in biotechnology that leverages an advanced polymer-based delivery platform, is creating unique nano formulations to be applied across various therapeutic sectors. Today, the company has revealed encouraging preliminary findings from a Phase I evaluation of ACM-001, their inaugural clinical-stage development initiative.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
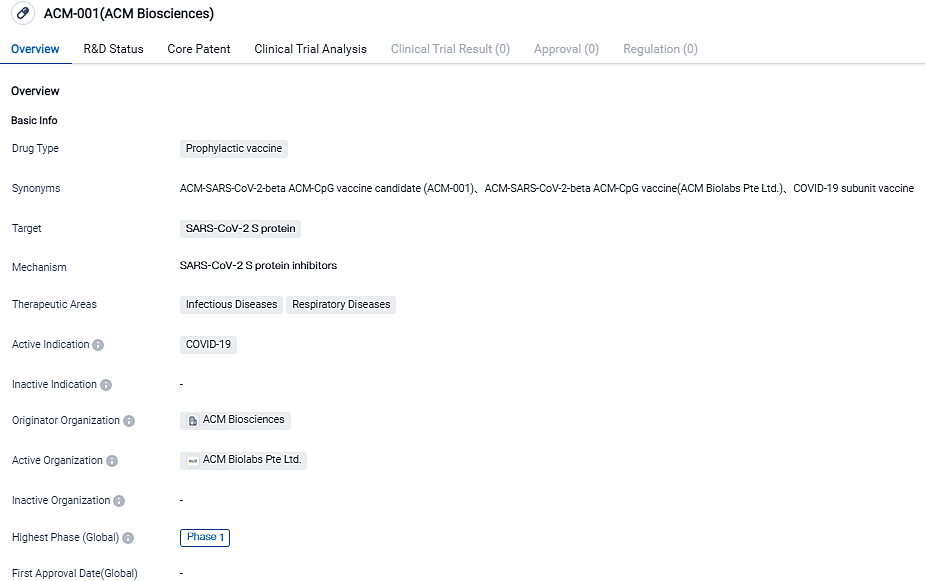
The ACM-001 is a supportive vaccine for SARS-CoV-2, the pathogen behind COVID-19, enhanced with an adjuvant. It is composed of a spike protein segment from the beta SARS-CoV-2 variant, known for evading immune responses, and an immune booster, CpG 7909, engineered using the company's polymer-based delivery system, ACM Biolabs' Adjustable Platform.
A study involving 36 participants across six Australian locations demonstrated the vaccine to be safe and well-tolerated at the suggested dosage, whether administered via standard intramuscular injection or mucosal delivery. Preliminary immunogenicity results point to a wide-ranging antibody response initiated by the intramuscular vaccine, targeting both past and presently circulating omicron variants.
Many recognized difficulties must be confronted by vaccine creators using lipid nanoparticles. The need for super-cold storage across the distribution network and a rigid processing timeframe are among these challenges. ATP™ has the potential to address many of these issues and ACM Biolabs also benefits from a solid and defined IP standing, supportive of its technology.
CEO of ACM Biolabs, Madhavan Nallani, Ph.D., commented, "This is outstanding for ACM Biolabs as it significantly reduces risk to our fundamental technology and empowers us to move forward with all our formulations in research and development. Our technology has garnered much interest from collaborators who we believe will find the data beneficial as we advance joint projects."
Pierre M.D., Ph.D., ACM Biolabs' Chief Medical Officer, added, "The positive Phase I results substantiate our adjustable proprietary delivery platform, giving us confidence to forge ahead and delve into the full spectrum of the technology's capacity across multiple therapeutic areas, including vaccines."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
 According to the data provided by the Synapse Database, As of September 6, 2023, there are 434 investigational drugs for the SARS-CoV-2 S protein, including 32 applicable indications, 401 R&D institutions involved, with related clinical trials reaching 994, and as many as 801 patents.
According to the data provided by the Synapse Database, As of September 6, 2023, there are 434 investigational drugs for the SARS-CoV-2 S protein, including 32 applicable indications, 401 R&D institutions involved, with related clinical trials reaching 994, and as many as 801 patents.
ACM-001, a preventative immunization contrived by ACM Biosciences, is designed to attack the S protein of the SARS-CoV-2 virus. It aims to stop COVID-19 and is classified in the medical fields of Infectious and Respiratory Diseases. Presently, ACM-001 is in the first stage of clinical trials, denoting progression in human experimentations.