Adicet Bio Showcases Preclinical Readiness of ADI-270 for IND at 27th ASGCT Meeting
Adicet Bio, Inc. has revealed that a summary discussing fresh preclinical results, which emphasize the potential of ADI-270, a fortified allogeneic gamma delta CAR T cell therapy designed for CD70 positive cancers, has been chosen for a live discussion. This presentation is scheduled for May 10, 2024, during the Targeted Gene and Cell Therapy segment at the 27th ASGCT Annual Meeting, which will be held from May 7-11, 2024, in Baltimore, MD. The session will be jointly presided over by Dr. Blake Aftab, the Chief Scientific Officer of Adicet Bio.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"We are eager to present ongoing preclinical findings at ASGCT, which underscore the powerful anti-tumor properties of ADI-270 using a specialized approach in targeting CD70 among various solid and hematologic malignancies," stated Blake Aftab, Ph.D., Chief Scientific Officer at Adicet Bio.
The results of this research enhance our understanding and provide a relative assessment of the ways ADI-270 achieves superior effectiveness and strength in cancers expressing CD70, such as clear cell renal cell carcinoma, and promote a strong anti-tumor response that justifies its further development. The preclinical results reveal:
In diverse cell cultures of CD70 negative and CD70 positive tumors, ADI-270 showcased potent cytotoxic effects, underscoring the capability of gamma delta CAR T cells to target tumors displaying a range of antigen profiles.
Employing a CD27-based strategy for CD70 targeting, ADI-270 exhibited intense CAR-mediated activity in several types of cancer including ccRCC, non-small cell lung cancer, and T cell lymphoma, and it was effective even in models with lower CD70 expression levels.
ADI-270 obstructed tumor growth within a suppressive tumor microenvironment due to the integration of a dominant-negative transforming growth factor beta receptor, and it proved resilient to elimination by host T cells owing to the properties of CD27-based CAR that targets CD70 present also on host T cells.
In an in vivo model of ccRCC, marked anti-tumor responses were noted post-treatment, including tumor infiltration, cell proliferation, and effector mechanics, culminating in the elimination of CD70 positive tumor cells.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
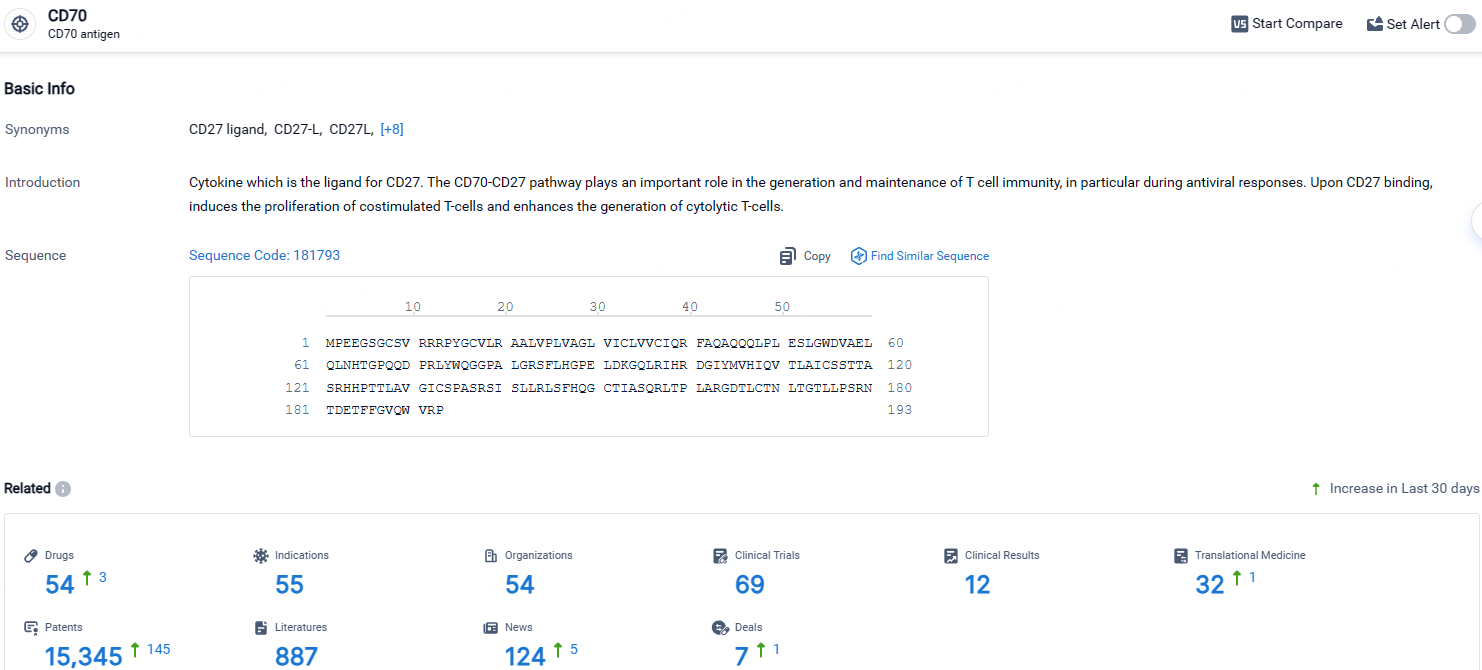
According to the data provided by the Synapse Database, As of April 24, 2024, there are 54 investigational drugs for the CD70 targets, including 55 indications, 54 R&D institutions involved, with related clinical trials reaching 69, and as many as 15345 patents.
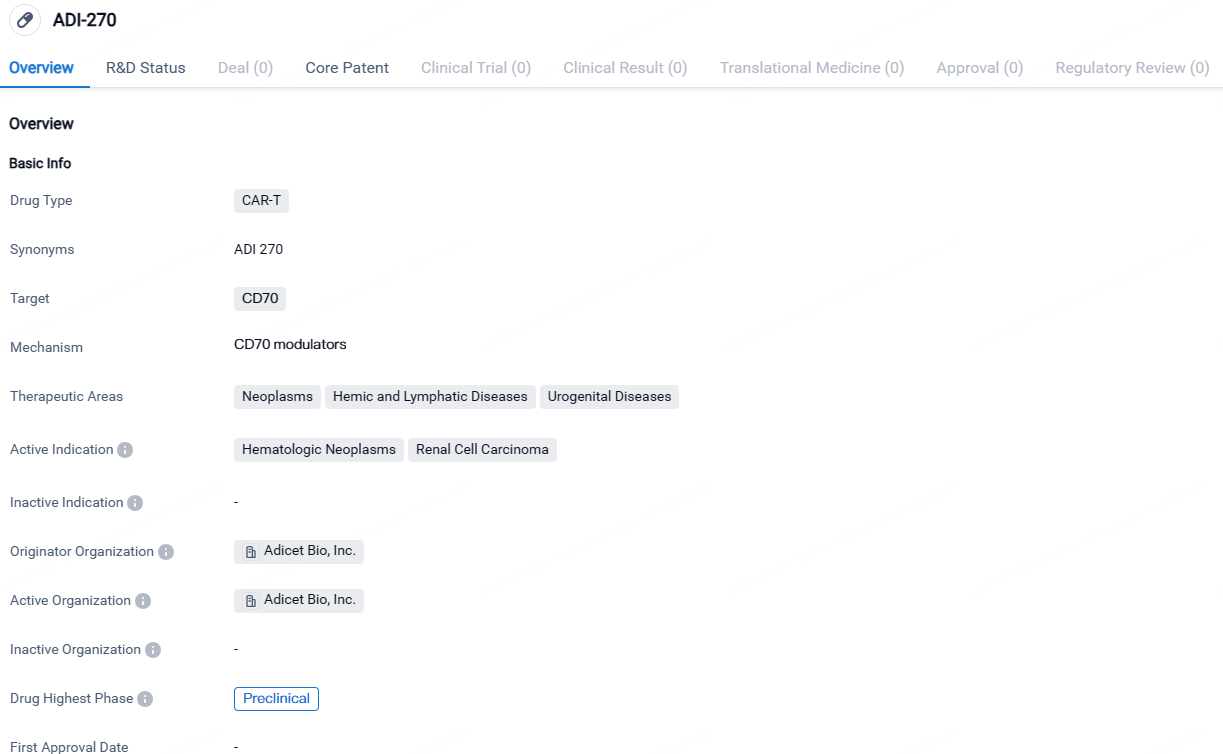
ADI-270 targets CD70 and is indicated for hematologic neoplasms and renal cell carcinoma. Currently, it is in the preclinical phase of development, and further studies are needed to determine its efficacy and safety in human subjects.