ASLAN Pharmaceuticals Announces Positive Early Results from Phase 2 Eblasakimab Trial in Atopic Dermatitis Patients Previously on Dupilumab
ASLAN Pharmaceuticals, a biopharmaceutical firm at the clinical stage with a focus on immunology, today disclosed encouraging preliminary outcomes from the TREK-DX Phase 2 trial, which examines eblasakimab in adults with moderate-to-severe atopic dermatitis who had prior treatments with dupilumab. The company specializes in creating novel therapies aimed at enhancing patient health.
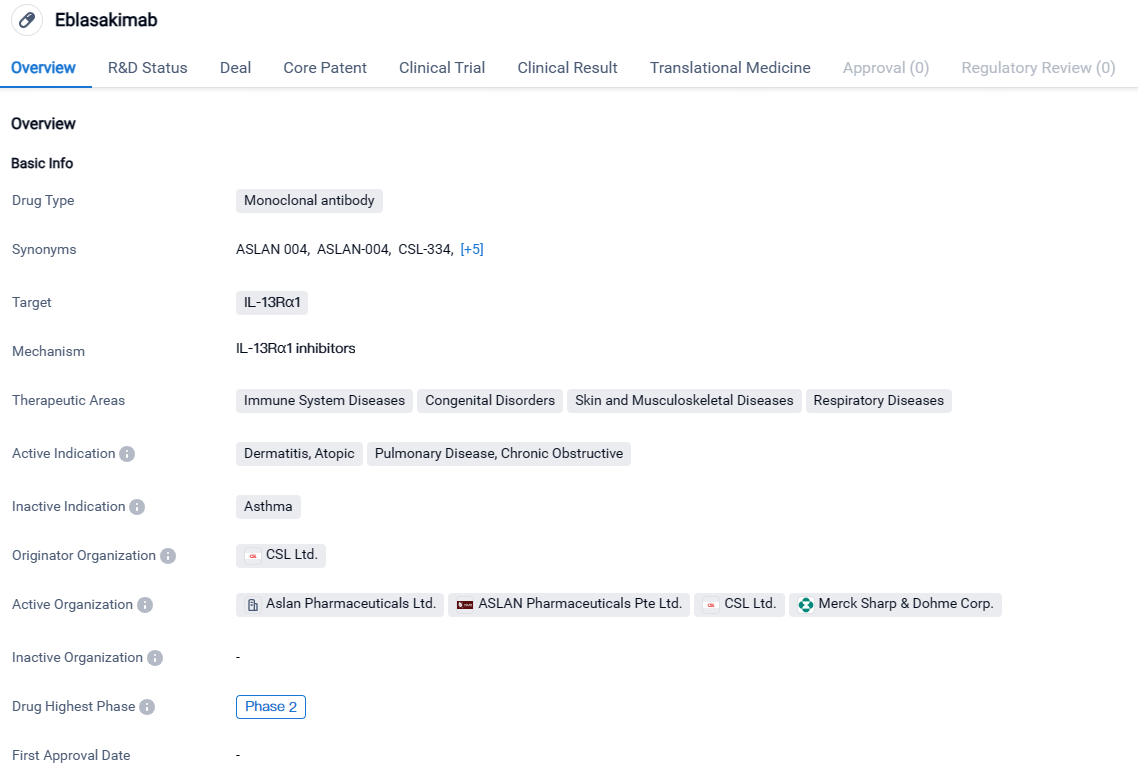
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The primary outcome of the study, the change in Eczema Area Severity Index (EASI) score from the start to week 16, demonstrated significant differences when compared with the control group receiving placebo, despite the early analysis not being statistically significant owing to the size of the sample group. A 73.3% majority of patients treated with eblasakimab saw their EASI scores decrease by at least 75% from initial levels, in contrast to only 14.3% of those receiving a placebo.
Dr. Carl Firth, CEO of ASLAN Pharmaceuticals, expressed great satisfaction with the outcomes. "The results from eblasakimab in higher dosing regimens surpass previous studies. The majority of eblasakimab participants reached both EASI-90 and vIGA scores of 0 or 1 within 16 weeks, a milestone not seen in other biologic studies for AD. Eblastakimab was particularly effective in patients unresponsive to prior dupilumab treatment, with two-thirds reaching EASI-90 and vIGA scores of 0 or 1."
Dr. Firth also added, "The anticipated year-end announcement of the comprehensive results from the TREK-DX study, a pioneering placebo-controlled trial involving AD patients previously treated with dupilumab, will be a significant milestone. We also aim to refine the dosing approach for eblasakimab in the upcoming Phase 3 trials."
Eblasakimab, a pioneering monoclonal antibody directed at the IL-13 receptor subunit of the Type 2 receptor, holds promise as an innovative treatment for various allergic inflammatory diseases by disrupting a crucial inflammatory pathway. Its distinct mode of action is designed to enhance the efficacy over existing biologics that manage these conditions.
By targeting the Type 2 receptor, eblasakimab interferes with the inflammatory signals mediated by interleukin 4 and interleukin 13, pivotal in the pathogenesis of AD and Type 2-driven Chronic Obstructive Pulmonary Disease (COPD). In July 2023, positive findings from the TREK-AD Phase 2b study examining eblasakimab in patients with moderate-to-severe, biologic-naïve AD were announced by ASLAN. The current TREK-DX Phase 2 trial is evaluating eblasakimab in moderate-to-severe AD patients experienced with dupilumab.
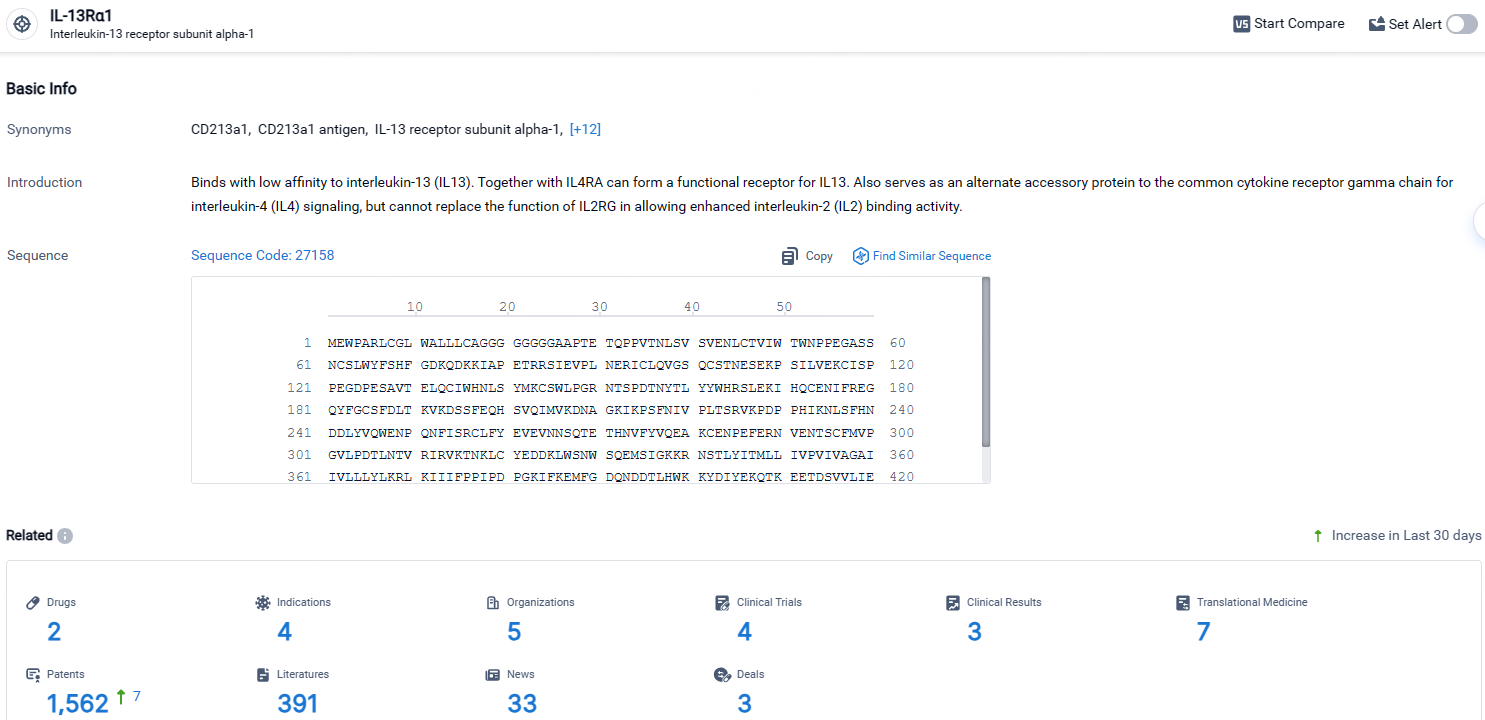
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of April 23, 2024, there are 2 investigational drugs for the IL-13Rα1 target, including 4 indications, 5 R&D institutions involved, with related clinical trials reaching 4, and as many as 1562 patents.
Eblasakimab targets IL-13Rα1 and has shown potential in treating immune system diseases, congenital disorders, skin and musculoskeletal diseases, and respiratory diseases. Currently in Phase 2, Eblasakimab is still undergoing clinical trials to determine its safety and efficacy. If successful, it could provide new treatment options for patients in need.