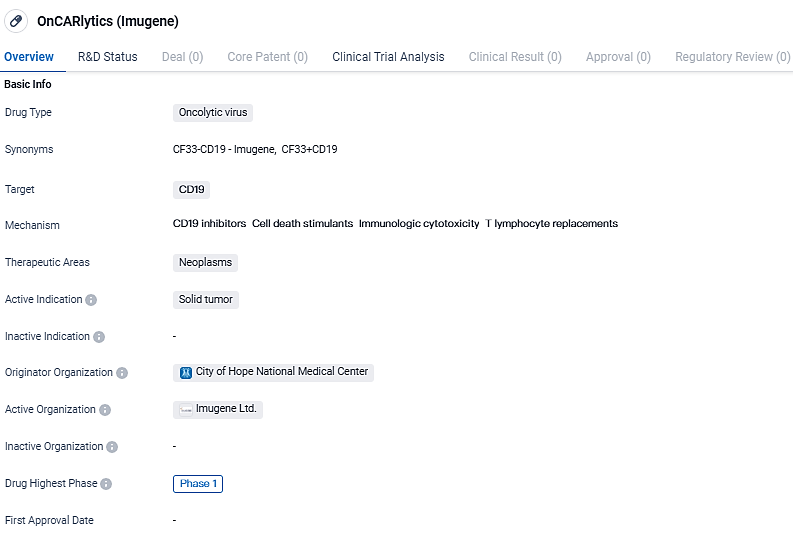
Advancing the onCARlytics Study: Exploring Combined Therapies in Early-Stage Solid Cancers
Imugene Limited, an enterprise specializing in immuno-oncology at the clinical phase, is excited to communicate that it has successfully progressed through the initial group of participants in its Phase 1 trial, examining the CD19-targeting oncolytic virotherapy called onCARlytics (on-CAR-19, CF33-CD19 HOV4). This development within the stand-alone, intratumoral treatment segment indicates the readiness to start the next phase. This will involve the use of a combined therapeutic approach in patients with solid tumors, utilizing the onCARlytics in conjunction with the CD19-specific treatment, blinatumomab.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Under the acronym OASIS, a novel clinical trial is enrolling adult participants with advanced or metastatic solid tumours to ascertain the safety profile and therapeutic efficacy of an investigational treatment through two delivery methods: direct intratumoral injection and systemic intravenous infusion. Participants in the trial may receive the treatment as a single agent or jointly with the bispecific monoclonal antibody known as blinatumomab.
The official designation of this study is: “A Phase I, Ascending Dose, and Cohort Expansion Trial to Assess the Safety and Feasibility of onCARlytics Given Either Through an Intravenous Route or Directly into the Tumour in Conjunction with Blinatumomab for Adults with Progressive or Disseminated Solid Neoplasms.”
Within the combined treatment segment of the research, onCARlytics will be used in tandem with blinatumomab, which targets CD19. This novel approach with onCARlytics could revolutionize the management of solid tumours, providing a new option for patients who do not respond to Blincyto treatment alone.
The investigation, OASIS, is designed to incrementally increase doses in participants and will be carried out at various locations within the United States, with a plan to include 52 patients in the trial.
Leslie Chong, the Managing Director and CEO of Imugene, stated: "This marks another testament to the diligence and partnership of our research teams, as we notice continued progress and adherence to our timelines. With the initial phase focusing on monotherapy via intratumoral administration in subjects with ovarian, breast, and melanoma cancers now complete, we are poised to embark on a pivotal phase of joining onCARlytics with Blincyto. Our anticipation is high to witness the enhanced capability of onCARlytics in confronting and eliminating solid tumours."
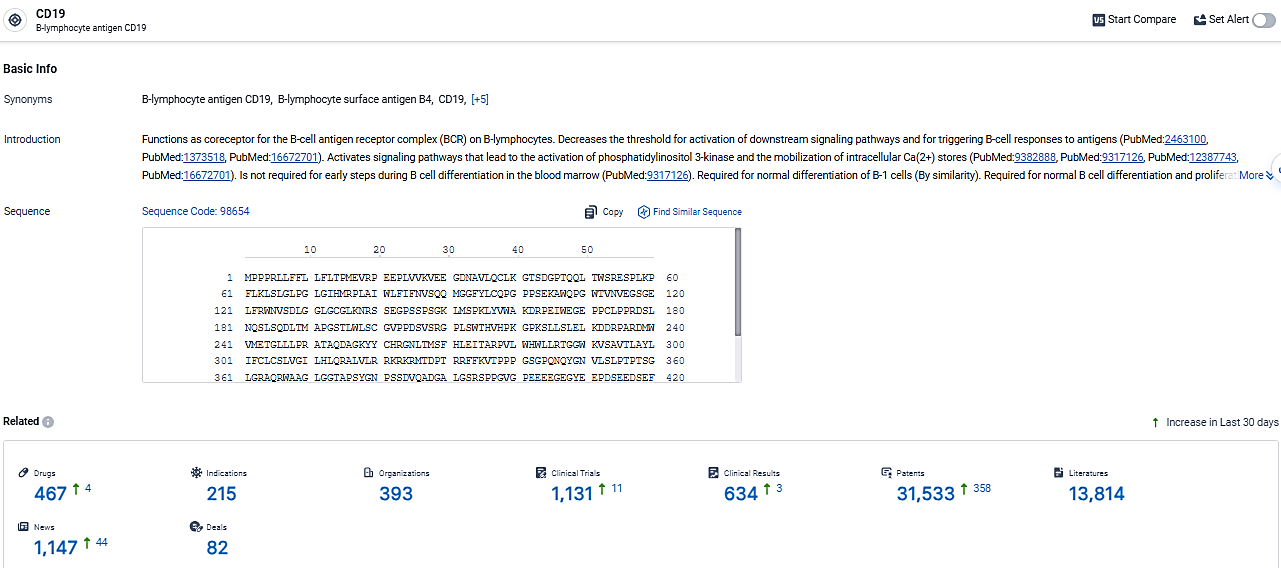
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 13, 2024, there are 467 investigational drugs for the CD19 target, including 215 indications, 393 R&D institutions involved, with related clinical trials reaching 1131, and as many as 31533 patents.
OnCARlytics t targets CD19 and is intended for the treatment of solid tumors. Currently in Phase 1, the drug's safety and effectiveness are being evaluated. Further research and clinical trials will be needed to determine its potential as a therapeutic option for patients with solid tumors.