Affimed Announces Encouraging Phase 1 Results for AFM28 in Treating R/R AML
Affimed N.V. (Nasdaq: AFMD), a clinical-stage immuno-oncology firm dedicated to restoring patients' natural cancer-fighting abilities, has revealed the oral presentation of AFM28 data at the 66th ASH Annual Meeting and Exposition. The findings from the inaugural Phase 1 human study of AFM28 indicated encouraging outcomes in relapsed/refractory acute myeloid leukemia (R/R AML), demonstrating clinical efficacy and a manageable safety profile at weekly doses up to 300 mg.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The trial involved 29 R/R AML patients who had received extensive prior treatments, spread across six different dose levels of AFM28. The median number of previous treatment regimens was two, and 86% of patients were classified as having an unfavorable risk profile based on the 2022 European LeukemiaNet (ELN2022) guidelines. AFM28 was given intravenously once weekly across six dose levels, from 25 mg to 300 mg. The treatment was well-tolerated, with infusion-related reactions (IRRs) being the most common treatment-emergent adverse events, occurring in 45% of patients. All IRRs were mild to moderate (Grade 1 or 2). One patient experienced Grade 1 cytokine release syndrome (CRS), and no neurotoxicity or immune-effector related side effects were observed.
Among the six patients treated at the 250 mg dose, one achieved complete remission (CR) and remained on treatment for 6.5 months. At the 300 mg dose level, among 10 evaluable patients, there was 1 CR and 3 CRi, resulting in a composite complete remission rate (CRcR) of 40%. Four out of 10 patients continue to receive treatment with the potential for response deepening.
"Achieving a 40% composite complete remission rate with AFM28 in R/R AML is a significant achievement, particularly in this challenging patient population. Notably, we observe activity regardless of mutational status, including in patients with unfavorable prognostic molecular profiles. The manageable safety profile lays the groundwork for further development of AFM28, either as a standalone agent or in combination therapies," stated Dr. Andreas Harstrick, MD, Chief Medical Officer at Affimed.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
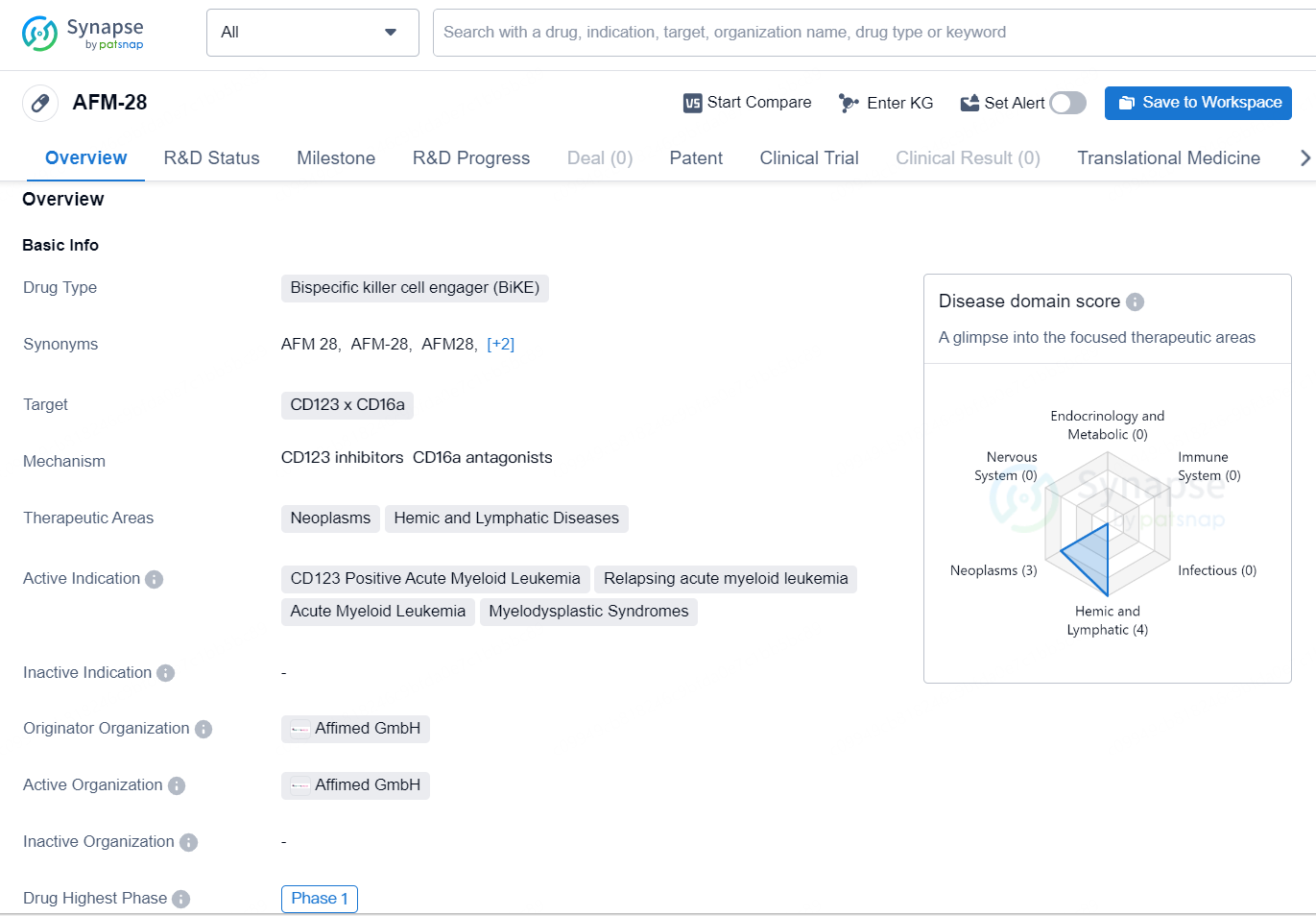
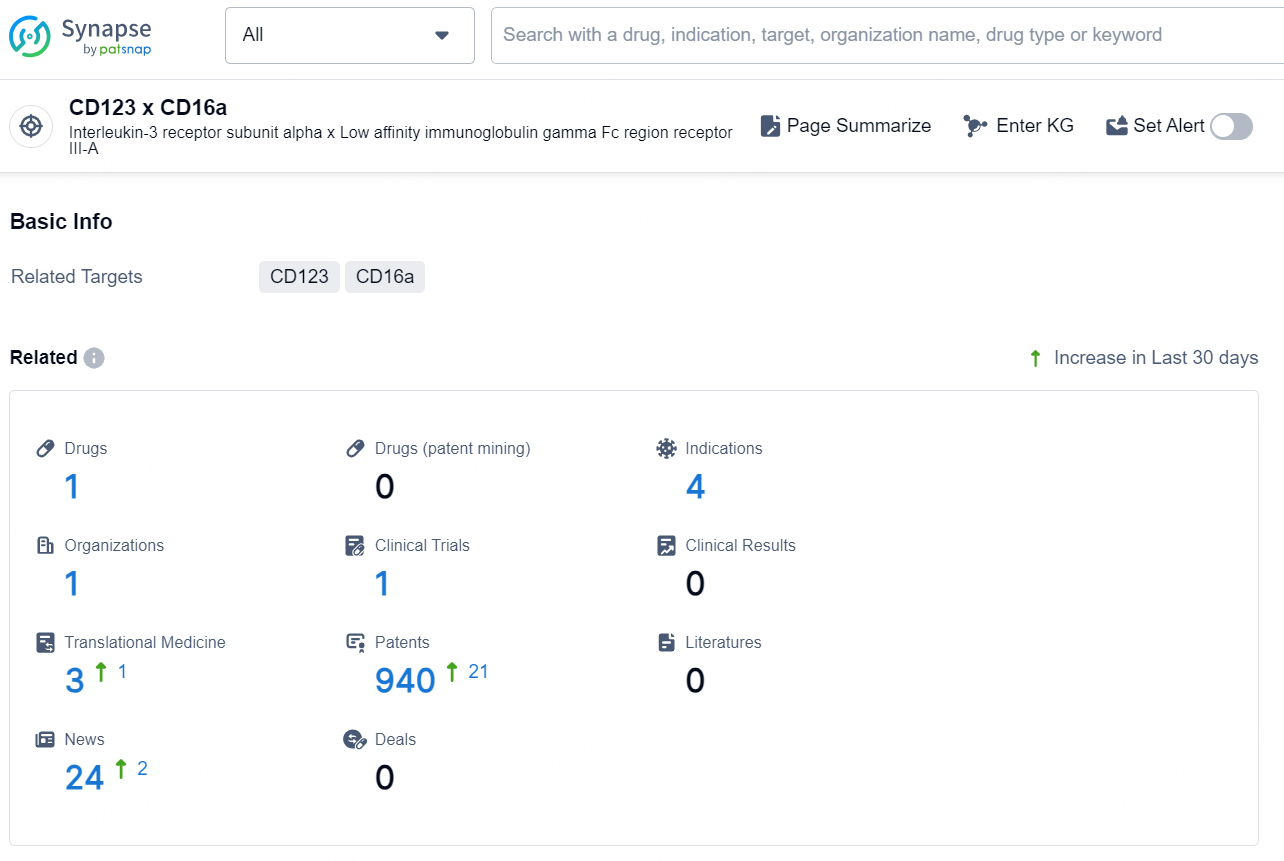
According to the data provided by the Synapse Chemical, As of December 13, 2024, there are 1 investigational drug for the CD123 x CD16a target, including 4 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 940 patents.
AFM-28 is a bispecific killer cell engager (BiKE) drug targeting the CD123 x CD16a, designed for the treatment of neoplasms, hemic, and lymphatic diseases. The active indications for AFM-28 include CD123 Positive Acute Myeloid Leukemia, relapsing acute myeloid leukemia, acute myeloid leukemia, and myelodysplastic syndromes.