Ajax Therapeutics Gains FDA Approval for New Myelofibrosis Drug AJ1-11095
Ajax Therapeutics, Inc., a company focused on biopharmaceuticals, has been granted approval from the U.S. Food and Drug Administration for its Investigational New Drug application, paving the way for a Phase I clinical trial of AJ1‑11095. This novel Type II JAK2 inhibitor is being developed primarily for the treatment of individuals suffering from myelofibrosis, as part of the company's advanced research into JAK inhibitors aimed at addressing myeloproliferative neoplasms.
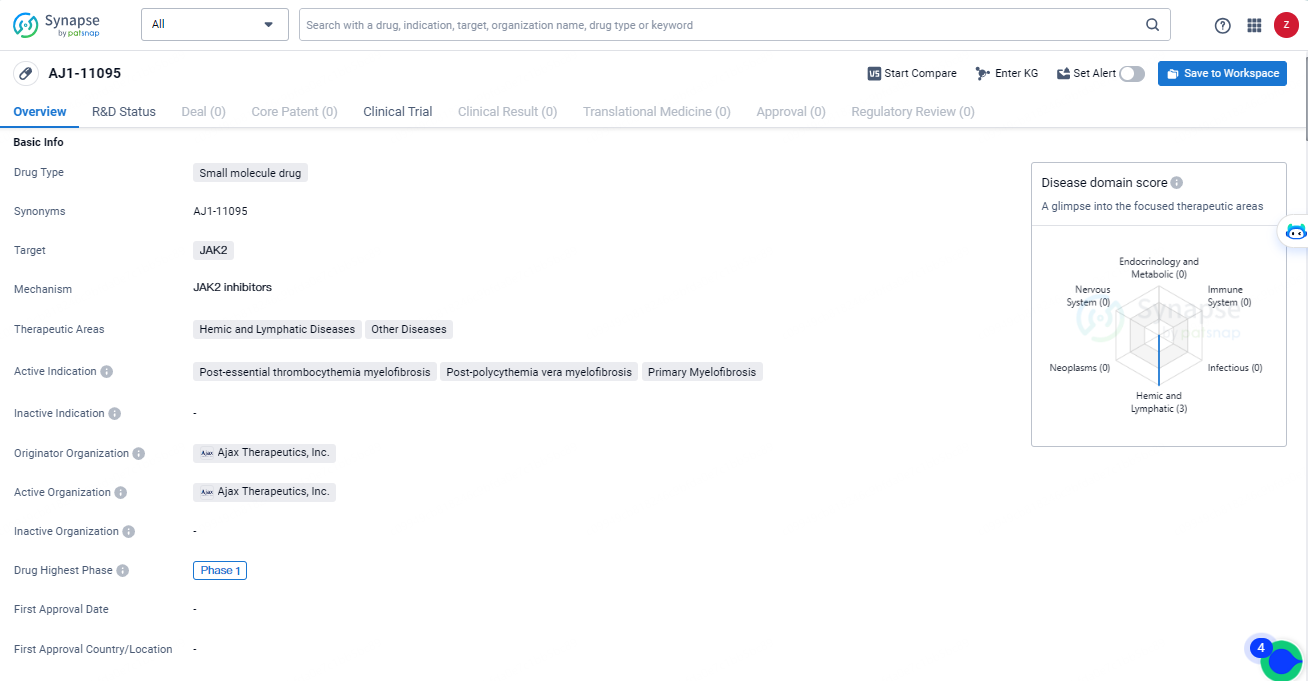
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Martin Vogelbaum, co-founder and CEO of Ajax Therapeutics, expressed his enthusiasm about the progression of AJ1-11095 into clinical trials, stating, "It's exhilarating to have the green light to move AJ1-11095 into clinical settings, offering hope with this novel therapeutic for those suffering from myelofibrosis.” He added, “This represents a significant achievement for Ajax Therapeutics as it marks both our inaugural clinical entry and the maiden clinical investigation to assess a Type II JAK2 inhibitor in this patient population."
David Steensma, MD, FACP, and Chief Medical Officer at Ajax, shared his anticipation for the future trials: “We are eager to commence the clinical trials for AJ1-11095 in myelofibrosis, specifically starting with the Phase 1 dose escalation study, AJX-101, scheduled for later this year.” He elaborated on the drug’s potential, noting, “AJ1-11095 introduces a groundbreaking approach as a Type II JAK2 inhibitor. It was specifically engineered to bring a superior option to the myelofibrosis treatments currently available, potentially enhancing efficacy and altering the course of the disease.”
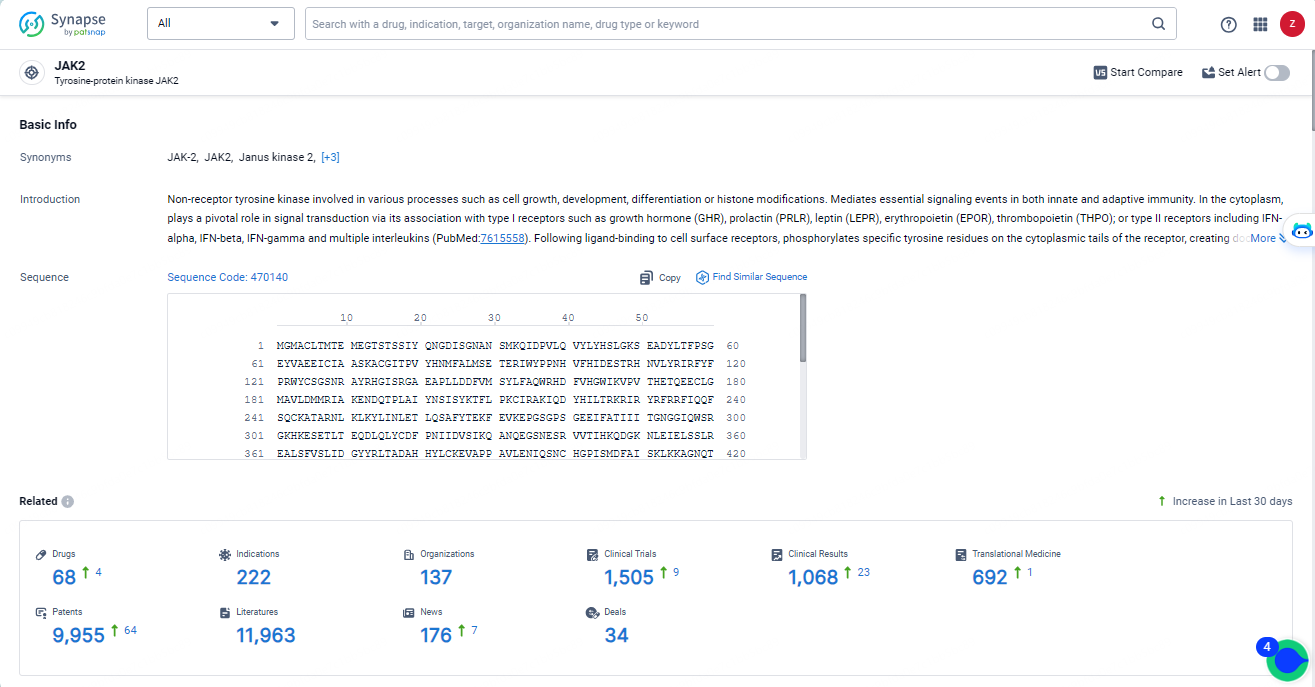
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 14, 2024, there are 68 investigational drugs for the JAK2 targets, including 222 indications, 137 R&D institutions involved, with related clinical trials reaching 1505, and as many as 9955 patents.
AJ1-11095 was designed to be a next generation JAK2 inhibitor by using structure-based drug design and computational methods at scale to selectively bind the Type II conformation of the JAK2 kinase and to provide greater efficacy with disease modification compared to all currently approved JAK2 inhibitors which bind the Type I conformation of JAK2. The drug's progression to higher phases of clinical development will provide more insights into its potential as a treatment option for patients with myelofibrosis and other related conditions.