FDA Prioritizes Review of Dupixent® for Teen Chronic Sinusitis and Nasal Polyps
Regeneron Pharmaceuticals, Inc. along with Sanofi have disclosed that the supplemental Biologics License Application for Dupixent® (dupilumab) has been given Priority Review status by the U.S. Food and Drug Administration. This application pertains to its use as a supplementary maintenance therapy for adolescents between the ages of 12 and 17 who suffer from chronic rhinosinusitis with nasal polyposis that is not adequately managed.
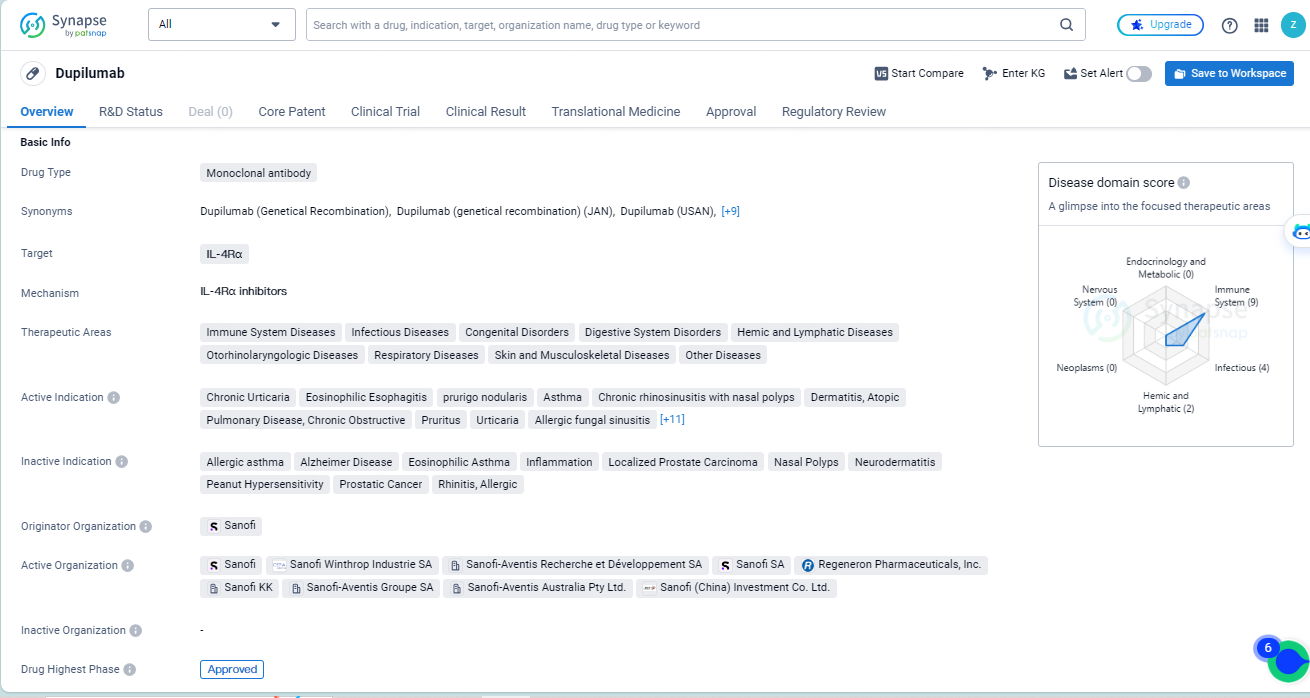
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
The FDA has set the review completion date for their decision on Dupixent as September 15, 2024. As of now, Dupixent is sanctioned for use in additional maintenance therapy for adult individuals with CRSwNP who do not experience sufficient disease control.
The sBLA submission for use in teenagers is bolstered by the efficacy extrapolation derived from two adult pivotal studies, which returned positive results in CRSwNP patients. In these studies, Dupixent significantly alleviated symptoms of nasal congestion and blockage, reduced the size of nasal polyps, enhanced olfactory perception, and lowered the dependency on systemic corticosteroids or surgical interventions within a six-month frame as compared to a placebo. Additionally, adolescent safety data for Dupixent in existing approved conditions further backed the sBLA.
In the SINUS-24 and SINUS-52 trials, the safety outcomes aligned closely with Dupixent’s well-established safety profile in previously approved uses. Notable adverse effects that occurred more frequently with Dupixent than with the placebo were reactions at the injection site and arthralgia.
A Priority Review designation is given to regulatory submissions that are judged to potentially offer substantial enhancements in the management, detection, or prevention of grave diseases. The consideration of Dupixent for treating adolescents with CRSwNP, however, remains under evaluation by health authorities.
Dupixent was developed using the cutting-edge VelocImmune® technology from Regeneron and is a fully human monoclonal antibody. It targets and inhibits the activity along the interleukin-4 (IL-4) and interleukin-13 pathways without acting as an immunosuppressant. Through its development phase, Dupixent has demonstrated compelling efficacy in decreasing type 2 inflammation during its Phase 3 studies, confirming that IL-4 and IL-13 are crucial mediators of the type 2 inflammatory process that significantly influences several interrelated conditions, often presenting comorbidities.
Globally, Dupixent has secured regulatory endorsements in over 60 countries for various indications, encompassing certain cases of atopic dermatitis, asthma, CRSwNP, eosinophilic esophagitis (EoE), prurigo nodularis, and chronic spontaneous urticaria across different patient age groups. Currently, Dupixent is used to treat over 850,000 patients worldwide.
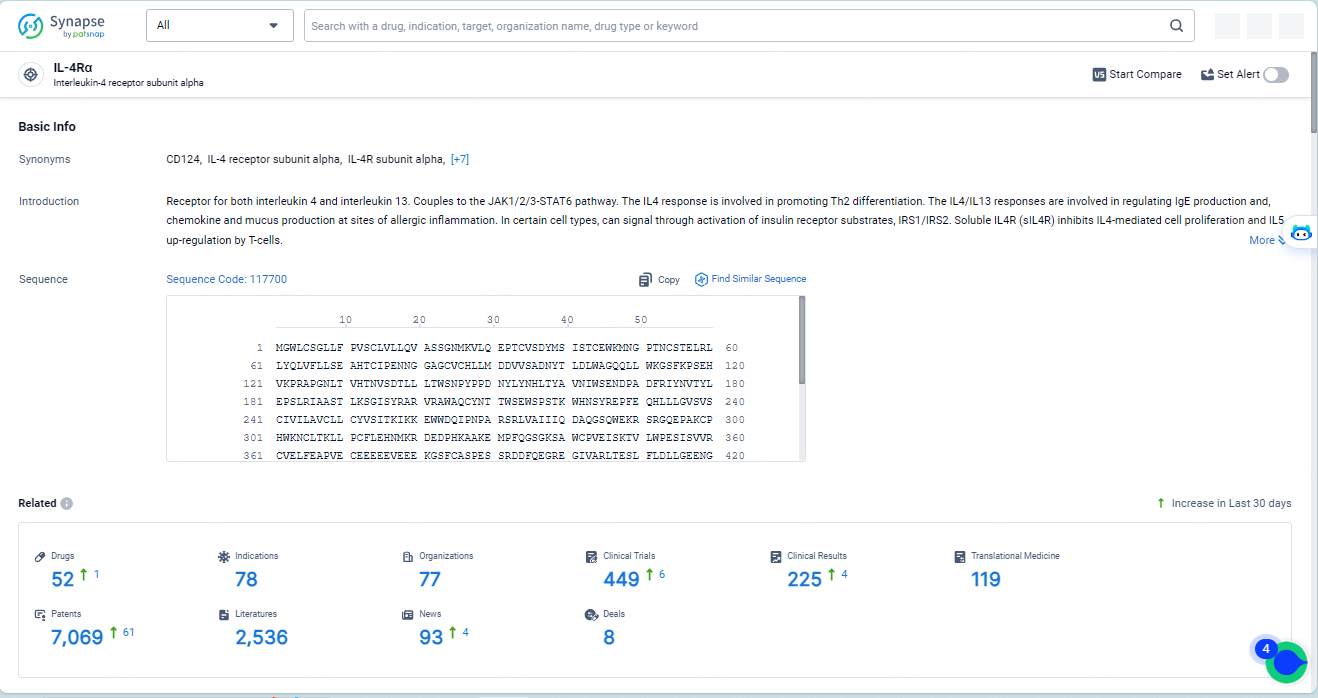
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 14, 2024, there are 52 investigational drugs for the IL-4Rα target, including 78 indications, 77 R&D institutions involved, with related clinical trials reaching 449, and as many as 7069 patents.
Dupilumab is a monoclonal antibody drug that targets IL-4Rα and has been approved for use in multiple therapeutic areas. It has shown efficacy in treating various indications and has received regulatory approvals in both the global and Chinese markets. The drug's development and approval have been supported by regulatory designations that emphasize its potential impact and urgency in addressing medical needs.