Alpine Immune Sciences Unveils Latest Trial Results for Povetacicept Treating IgA Kidney Disease
Alpine Immune Sciences, Inc., a pioneering enterprise at the clinical-development stage that concentrates on crafting cutting-edge immunotherapeutic strategies for the treatment of autoimmune disorders and inflammatory conditions, has disclosed new clinical findings relevant to povetacicept in the context of IgA nephropathy. These insights are slated for unveiling in the format of a late breaking poster during the World Congress of Nephrology, scheduled to occur from April 13 to 16, 2024, in Buenos Aires, Argentina.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Povetacicept demonstrates strong inhibition as a combined adversary to the BAFF and APRIL cytokines, essential contributors to the origin of diverse autoimmune disorders. This hinges on their crucial involvement in the activity, maturation and/or perseverance of B cells, especially those that produce antibodies, in concert with T cells and components of the innate immune system. The RUBY-3 clinical trial is designed as a multiple ascending dose, spread across different groups, with an open-label format. This phase 1b/2a trial investigates the effects of povetacicept in patients with autoimmune glomerulonephritis, which encompasses IgA nephropathy. Here, the drug is given subdermally in a four-week interval.
By March 1st, 2024, a cohort of 41 individuals battling IgAN had undergone treatment with either 80 mg or 240 mg of povetacicept, administered subdermally on a monthly schedule. Observations indicate that a dosage of 80 mg of povetacicept, given in a subcutaneous manner every four weeks, is linked to a noteworthy positive change in reducing proteinuria. Specifically, a 64.1% deduction from the initial urine protein to creatinine ratio (UPCR) has been recorded over a period of 36 weeks in a subgroup of six patients, whilst maintaining a consistent kidney function judged by the estimated glomerular filtration rate. At the same juncture, a 67% ratio (4 out of 6 individuals) reached a state of remission, characterized by a UPCR of less than 0.5 g/g, a decrease in UPCR by at least half from the outset, alongside a stable kidney function.
Jonathan Barratt, M.D., holding the title of Mayer Professor of Renal Medicine, also serving as an Honorary Consultant Nephrologist and Lead of the IgA Nephropathy Rare Disease Group at the University of Leicester, acknowledges the promising nature of these latest outcomes from povetacicept. He emphasizes the therapeutic advancement as being critical for IgA nephropathy treatment.
Delving deeper, Jonathan Barratt highlights the striking enhancements in UPCR and the consistent eGFR rates attained through the monthly treatment schedule at the 36-week mark. These improvements, coupled with considerable depletion of Gd-IgA1 — a paramount biomarker linked to the disease — and the resolution of blood in urine, stand out. The lack of severe adverse events, even after prolonged treatment periods and increased dosages, is noteworthy. Barratt asserts that, together with povetacicept's potential for altering the disease's trajectory and extensive patient experience, these factors collectively reinforce the rationale for fast-tracking povetacicept into a pivotal trial for addressing IgA nephropathy.
Echoing this sentiment, Stanford Peng, MD PhD, President and Head of Research and Development at Alpine, expresses renewed optimism for the prospects of povetacicept in IGAN and a spectrum of other autoimmune conditions.
Peng also shares satisfaction regarding the successful culmination of a phase 2 dialogue with the FDA, paving the way for a registration-directed trial in IgAN slated for later in the year. Furthermore, the intention to further assay povetacicept's therapeutic potential in a broader array of autoimmune illnesses through the ongoing RUBY-3 and RUBY-4 trials remains firm. He notes anticipation for the launch of a phase 2 study (named DENALI) assessing the drug in systemic lupus erythematosus, expected to commence within the year.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
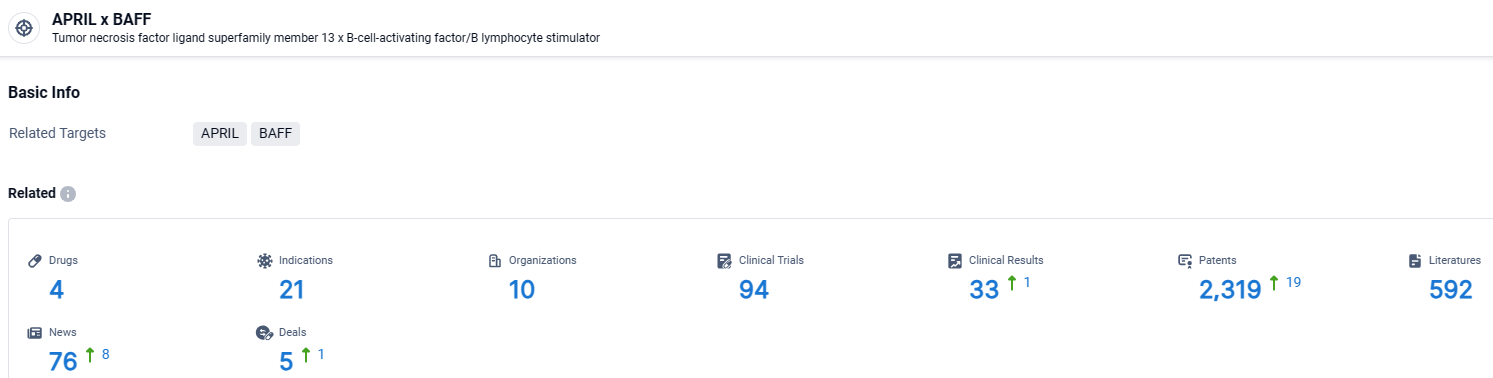
According to the data provided by the Synapse Database, As of April 15, 2024, there are 4 investigational drugs for the BAFF and APRIL target, including 21 indications, 10 R&D institutions involved, with related clinical trials reaching 94, and as many as 2319 patents.
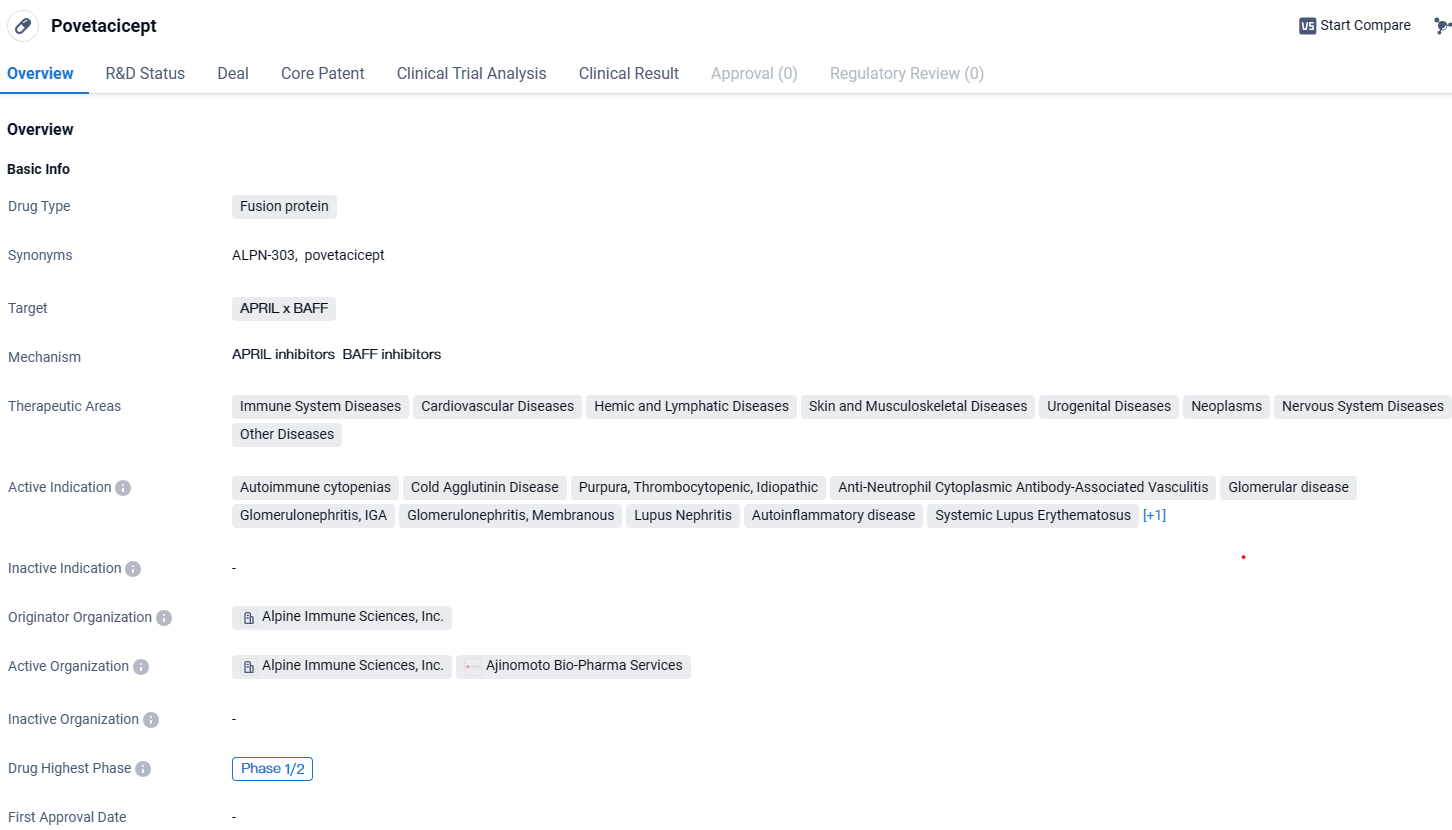
Povetacicept is a fusion protein drug developed by Alpine Immune Sciences, Inc. It targets APRIL x BAFF and has potential applications in various therapeutic areas, including immune system diseases, cardiovascular diseases, and neoplasms. The drug is currently in the early stages of development, with ongoing clinical trials to assess its safety and efficacy.