Amolyt Pharma and Core Peptide Patent Product Acquired by AstraZeneca
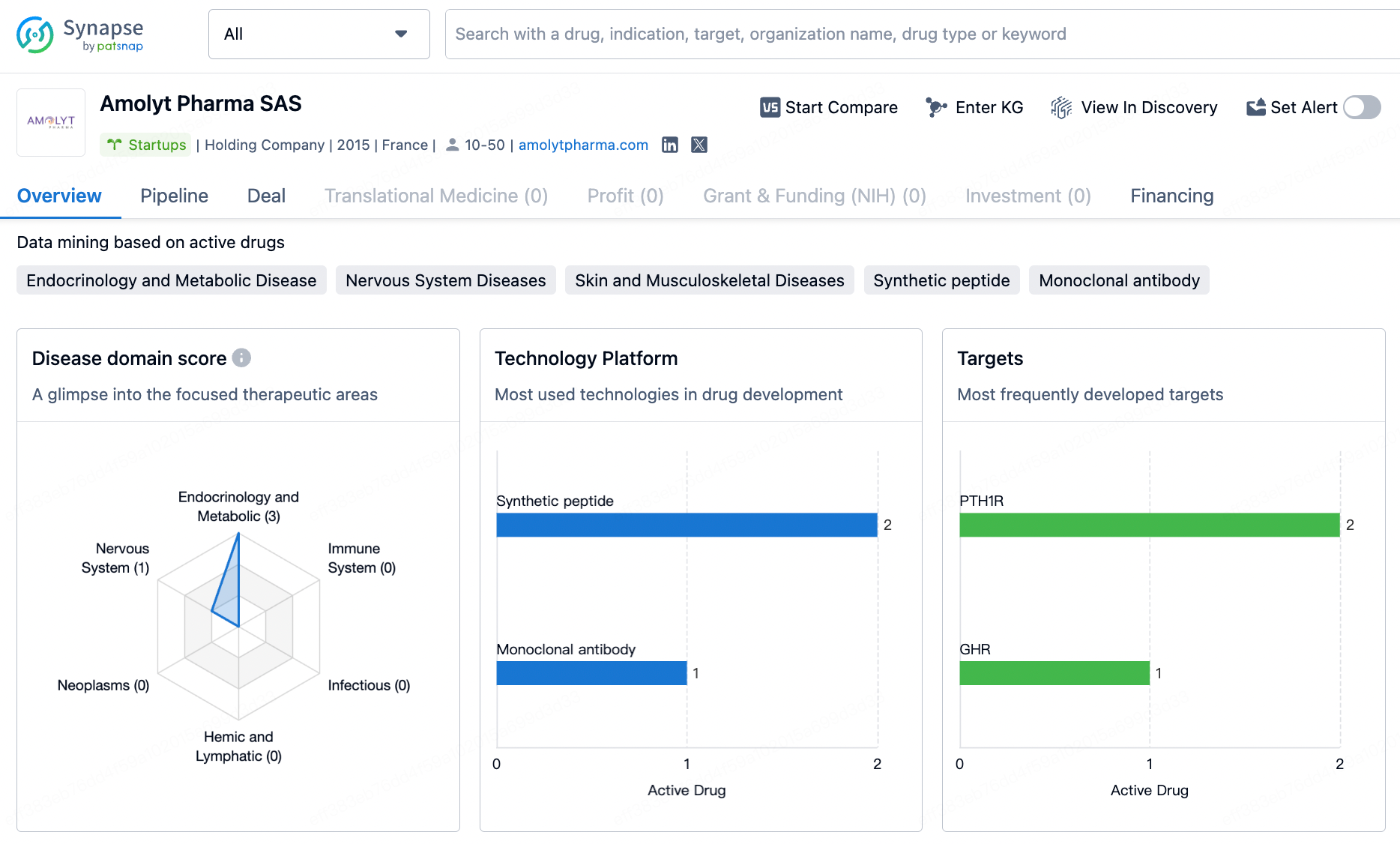
In March, British pharmaceutical giant AstraZeneca announced its intention to acquire Amolyt Pharma, a clinical-stage biotechnology company focused on developing innovative therapies for rare endocrine diseases. This acquisition will further strengthen AstraZeneca’s late-stage product development pipeline within its rare disease division, Alexion, and add a significant new element to its bone metabolism portfolio—the therapeutic peptide eneboparatide (AZP-3601), currently in Phase III clinical trials.
In 2018, Amolyt Pharma obtained the development rights for the long-acting peptide candidate drug AZP-3601 from The General Hospital Corp. AZP-3601 is a unique parathyroid hormone (PTH) analogue designed by researchers at The General Hospital Corp. for the treatment of hypoparathyroidism as a PTH replacement therapy. AZP-3601 interacts powerfully with a specific conformation of the PTH receptor, leading to prolonged activation and effect on calcium metabolism. Early studies have indicated that AZP-3601 is significantly more effective and has a longer-lasting effect in raising and maintaining blood calcium levels compared to natural PTH, without increasing urinary calcium excretion. The unique mechanism of action of AZP-3601 is expected to translate into improved safety and efficacy profiles, making it an optimal choice for the treatment of this rare but serious endocrine disease.
The PTH receptor is a G protein-coupled receptor that plays a pivotal role in regulating intracellular signaling pathways involved in bone growth and the homeostasis of blood calcium ions. Any disruption to the PTH receptor signaling system can be associated with a variety of bone diseases and abnormalities in mineral ion physiology. Dr. Gardella's research on the functional molecular mechanisms of the PTH receptor has revealed important clues that contribute to the development of new therapies for diseases such as Jansen's metaphyseal chondrodysplasia, hypocalcemia, and osteoporosis.
A patent from 2002 (US 6362163 B1) describes the properties of derivative peptides and mutants of the PTH peptide.
Under physiological conditions, PTH1R can be activated by two different endogenous peptide agonists (Parathyroid Hormone (PTH) and Parathyroid Hormone-related Protein (PTHrP)), to regulate multiple signaling pathways and exert unique biological effects. PTH secreted by the parathyroid glands primarily regulates the homeostasis of calcium and phosphate in the bones and kidneys, whereas PTHrP is involved in the proliferation and differentiation of cells in developing bone and other tissues.
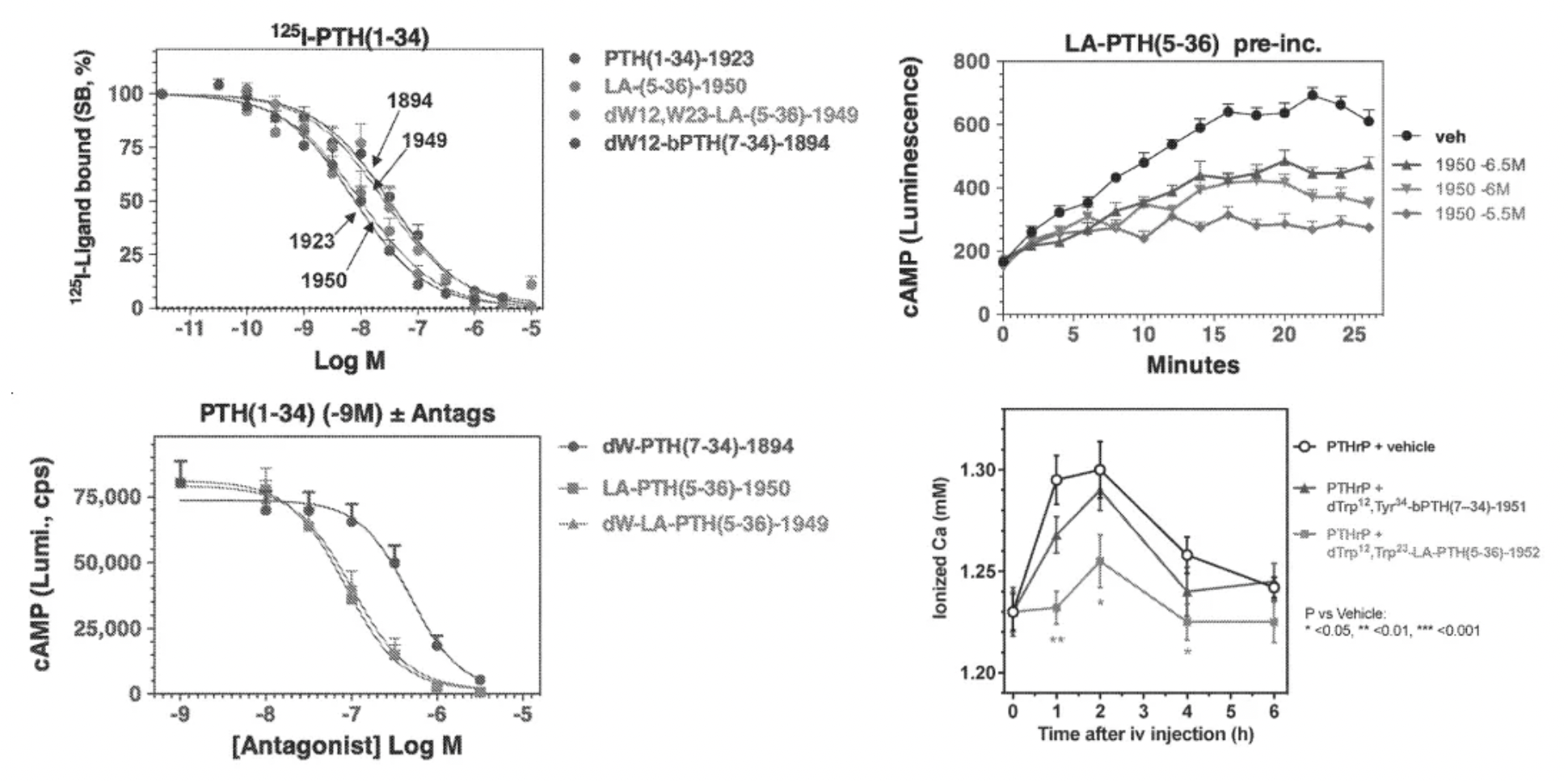
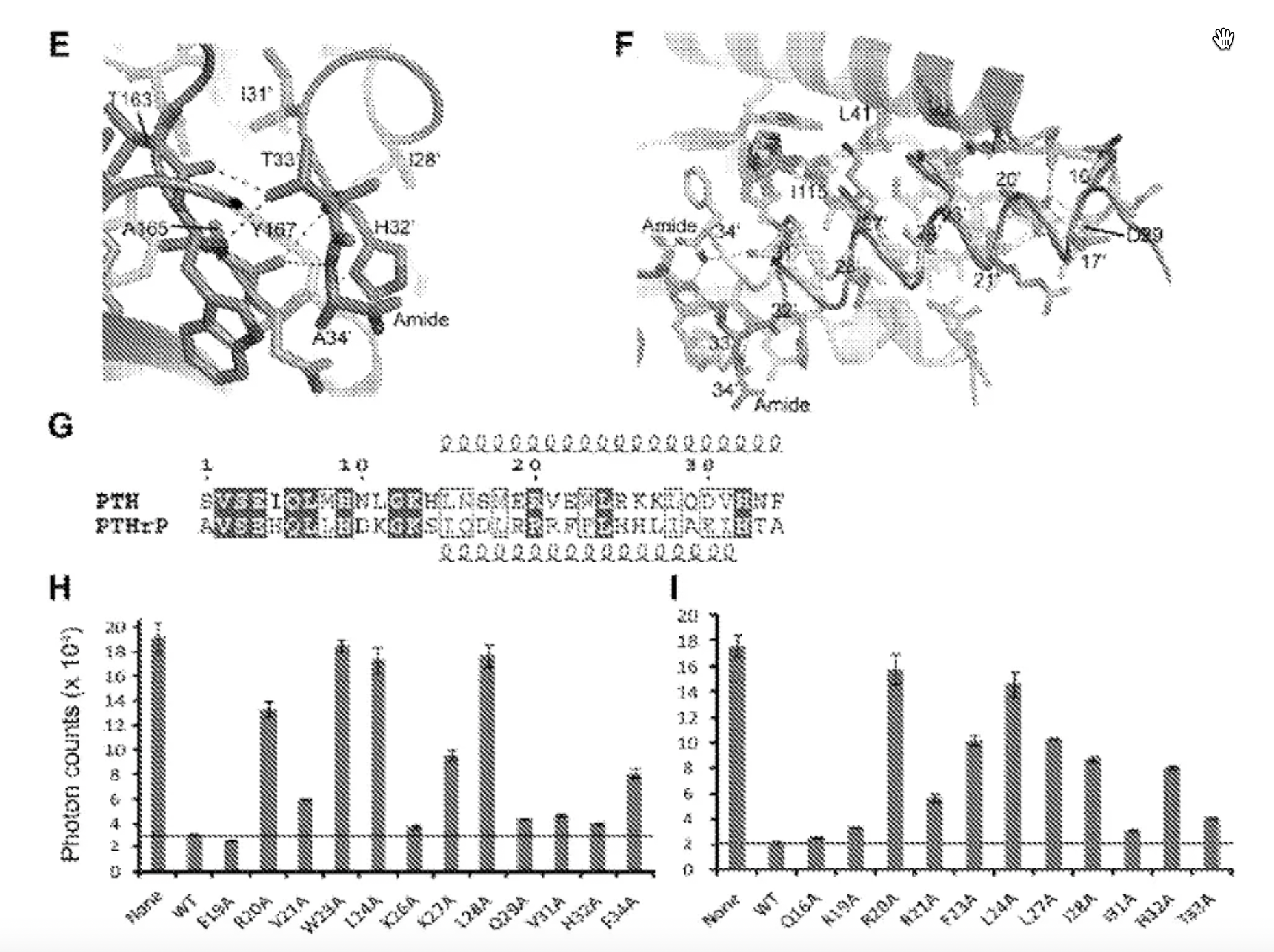
In this patent, researchers studied combinations of amino acid sequences at the N-terminus and C-terminus of PTH and PTHrP peptides and tested the selective activation of PTH and PTHrP peptides and their derivatives on PTH1R and PTH2R. The patent also tested substitutions at key amino acid positions 5 and 23, confirming the impact of these two amino acids on the specificity of the peptides.

A study in a patent from 2006 (US 7022815 B1) investigated mutant variants of PTH peptide derivatives and disclosed several peptide derivatives as well as pharmaceutical compositions containing peptides, along with methods to screen candidate compounds of the invention.
Researchers conducted mutational studies on the first 14 amino acids of the N-terminal of PTH peptides and tested the role of each residue in ligand recognition. Overall, mutations in certain N-terminal amino acids such as positions 4, 5, 6, 8, and 9 had a significant impact on the activity of the peptides.

A patent from 2009 (US61164284P0) studied the structural information related to PTH peptides and the derivatives of PTH peptide C-terminal mutants, as well as their role in regulating receptor activity. The invention involves peptides or polypeptides that activate the parathyroid hormone receptor and potential applications of these peptides or polypeptides in promoting bone growth (such as treating osteoporosis) or in cancer therapy.
A patent from 2016 (WO 2017/173372 A1) investigated antagonists or inverse agonist derivatives of PTH peptides. Overactive signaling of PTHR1 may be associated with higher than normal serum calcium levels, serum phosphate levels lower than normal, or constitutive activity of PTHR1 mutants. These peptides can be used in methods for treating conditions or diseases characterized by overactive PTH1R signaling.