An analysis of Apalutamide's R&D progress and its clinical results presented at the 2024 ASCO_GU Annual Meeting
Early PSA response has been found to be associated with improved outcome in mHSPC patients treated with androgen deprivation therapy (ADT) plus androgen receptor pathway inhibitors (ARPI). Recently, the 2024 ASCO_GU updated the treatment effect on OS varies.
Apalutamide's R&D Progress
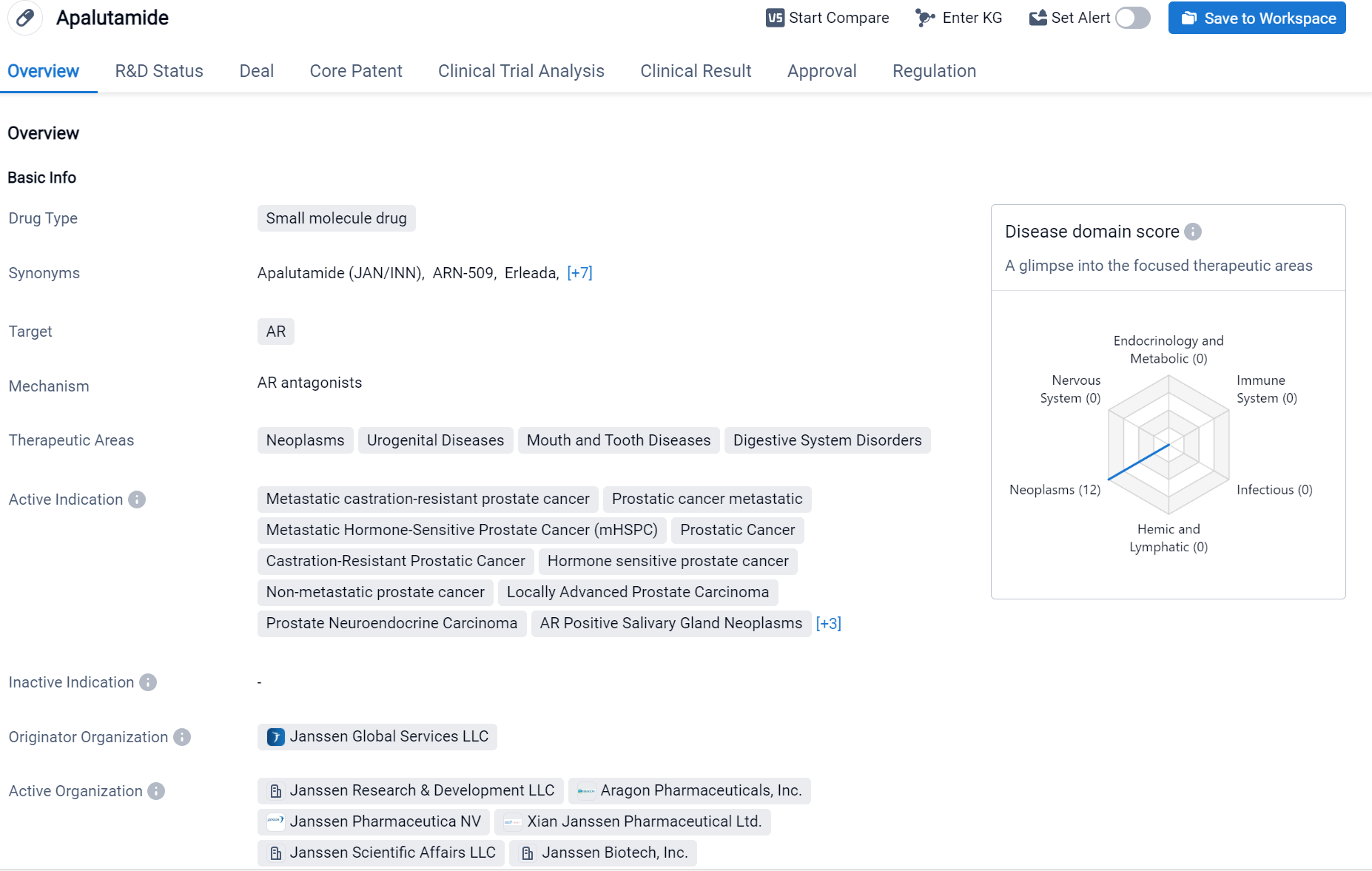
Apalutamide is a small molecule drug that targets the androgen receptor (AR). It is primarily used in the treatment of various neoplasms, urogenital diseases, mouth and tooth diseases, digestive system disorders, and liver diseases.
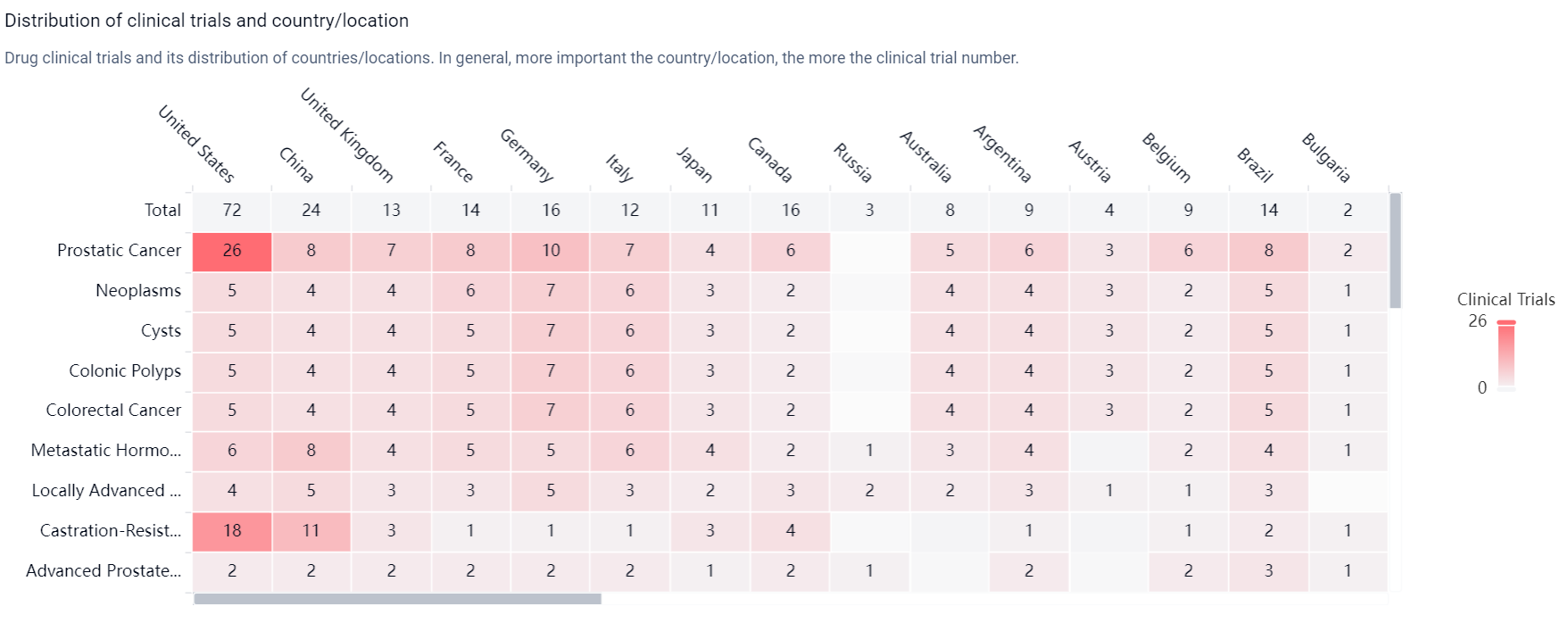
According to the Patsnap Synapse, Apalutamide was first approved in the United States in February 2018 by the originator organization, Janssen Global Services LLC. It has also received approval in China. And the clinical trial distributions for Apalutamide are primarily in the United States, China and United Kingdom. The key indication is Prostatic Cancer.
Detailed Clinical Result of Apalutamide
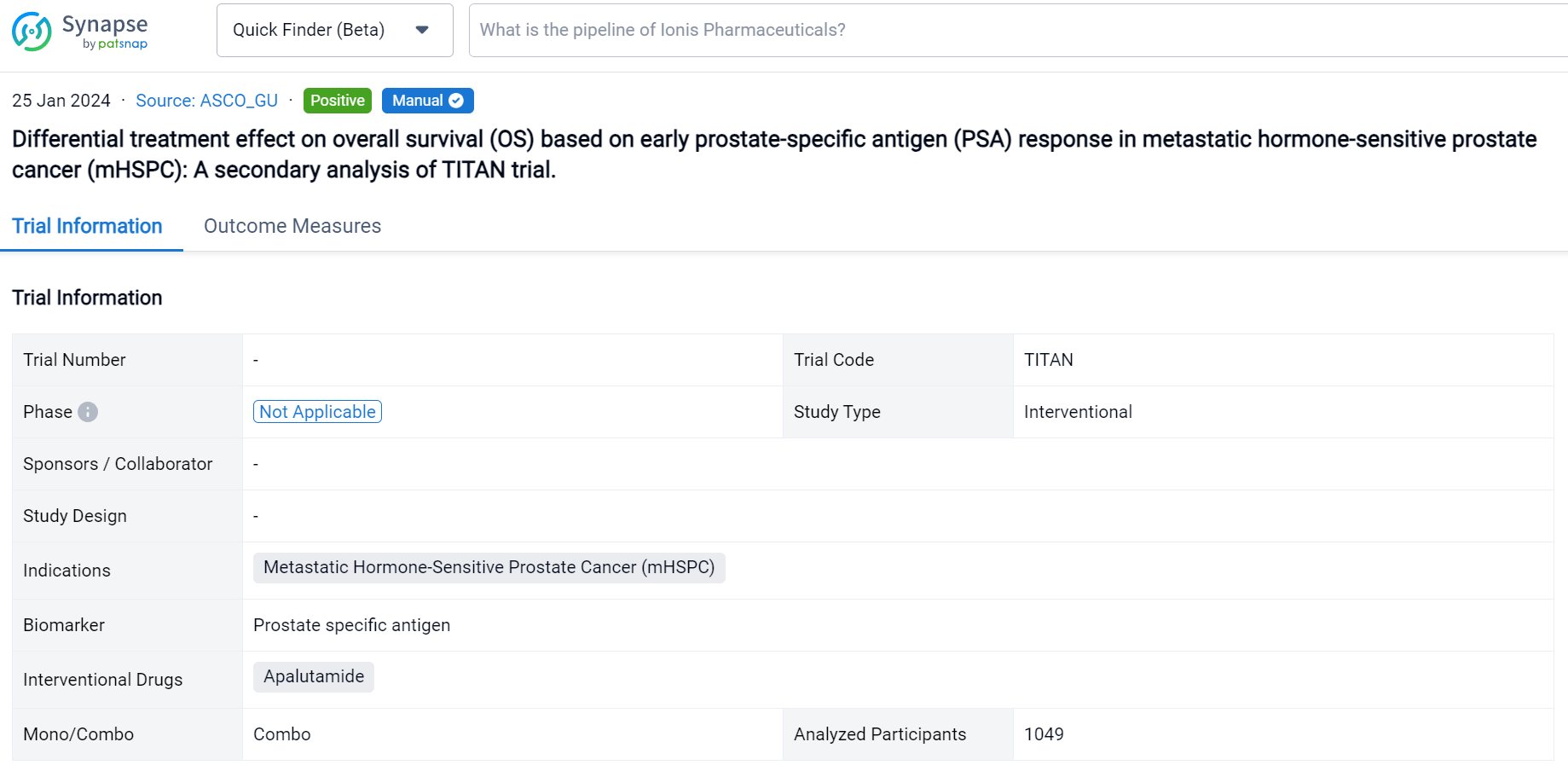
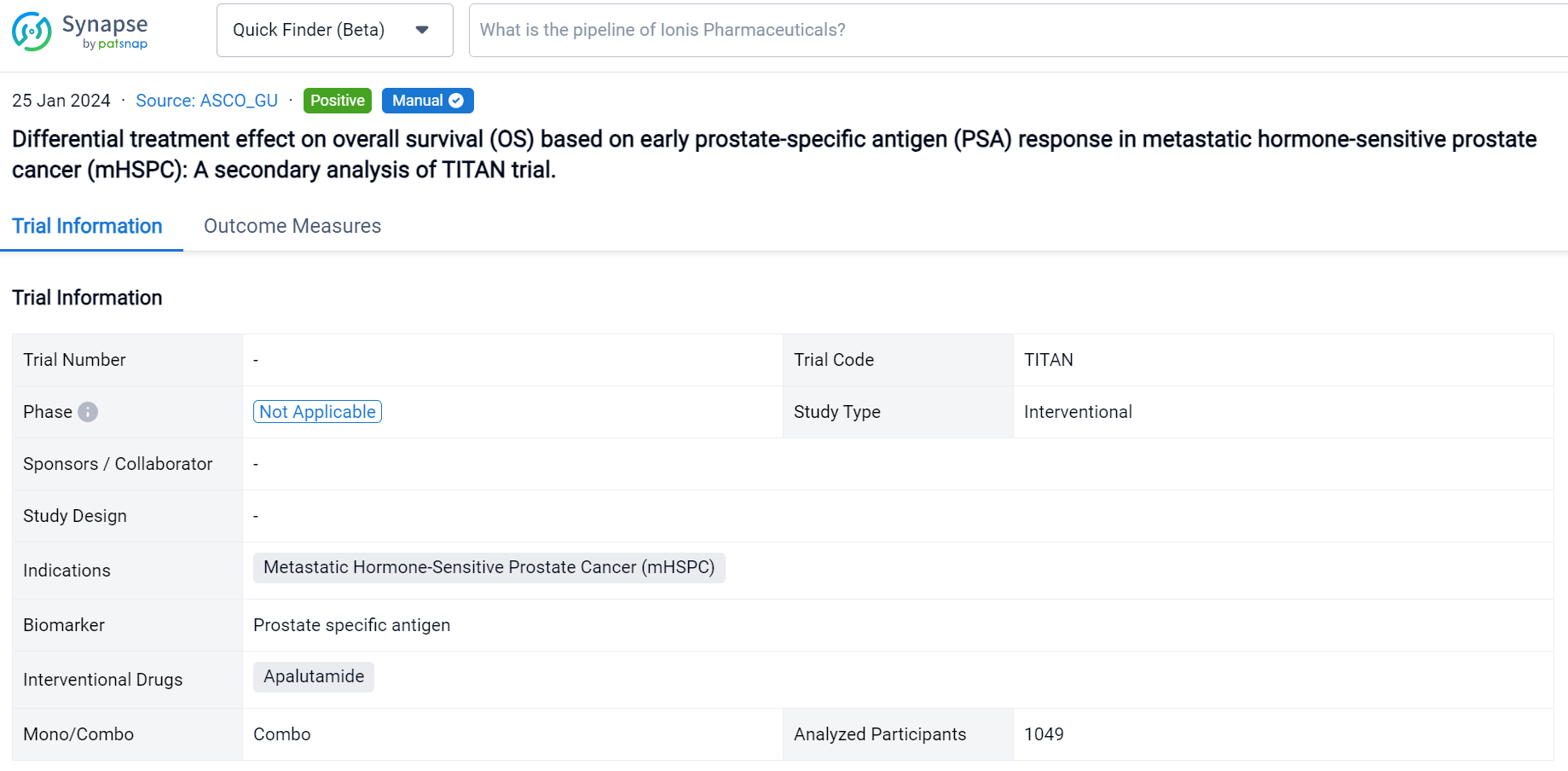
This secondary analysis of TITAN trial was aimed to explore the treatment effect (ADT) plus ARPI) on OS varies based on early PSA response in mHSPC patients.
In this study, the authors performed a secondary analysis of TITAN study in which men with mHSPC were randomly assigned to ADT plus placebo vs. ADT plus apalutamide (ADT+APA). To compare the association of OS with early PSA response, defined as achieving a PSA nadir of ≤0.2 ng/mL at ≤6 months of randomization, between two arms, we applied multivariable Cox regression model on a landmark population with an interaction term between the treatment arm and PSA response. Adjusted OS were calculated. Confounders were chosen based on their association with PSA response and OS.
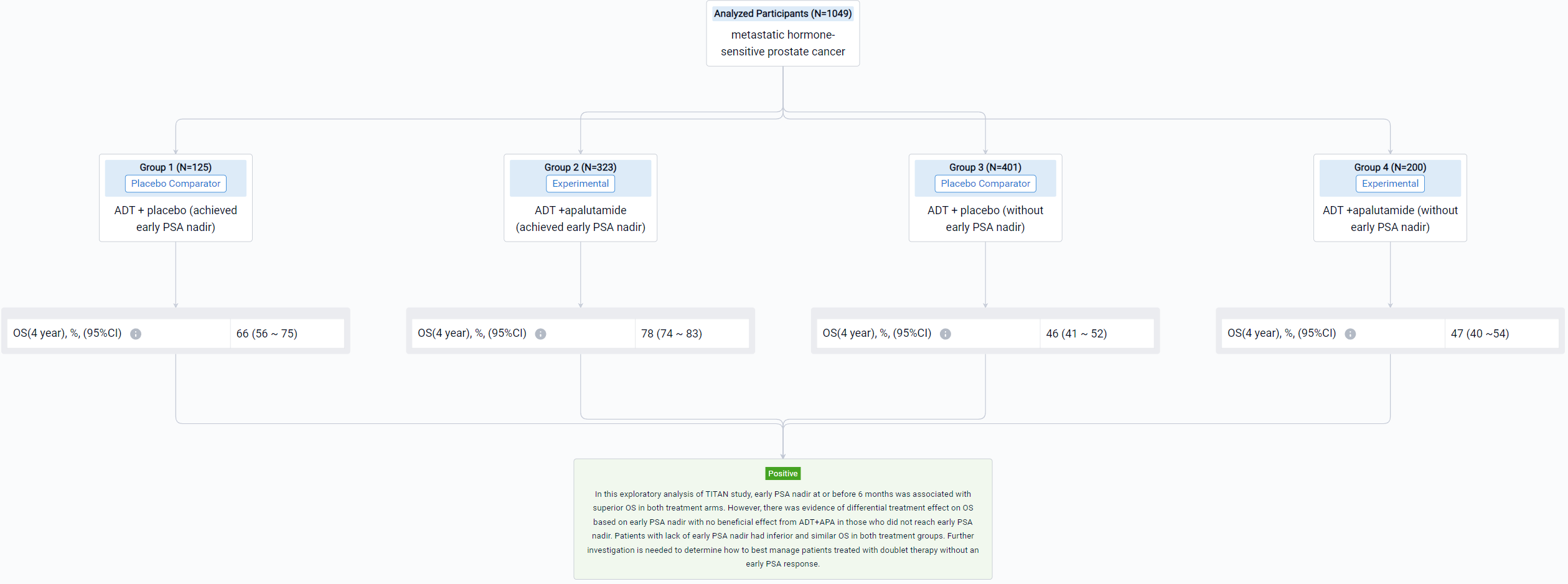
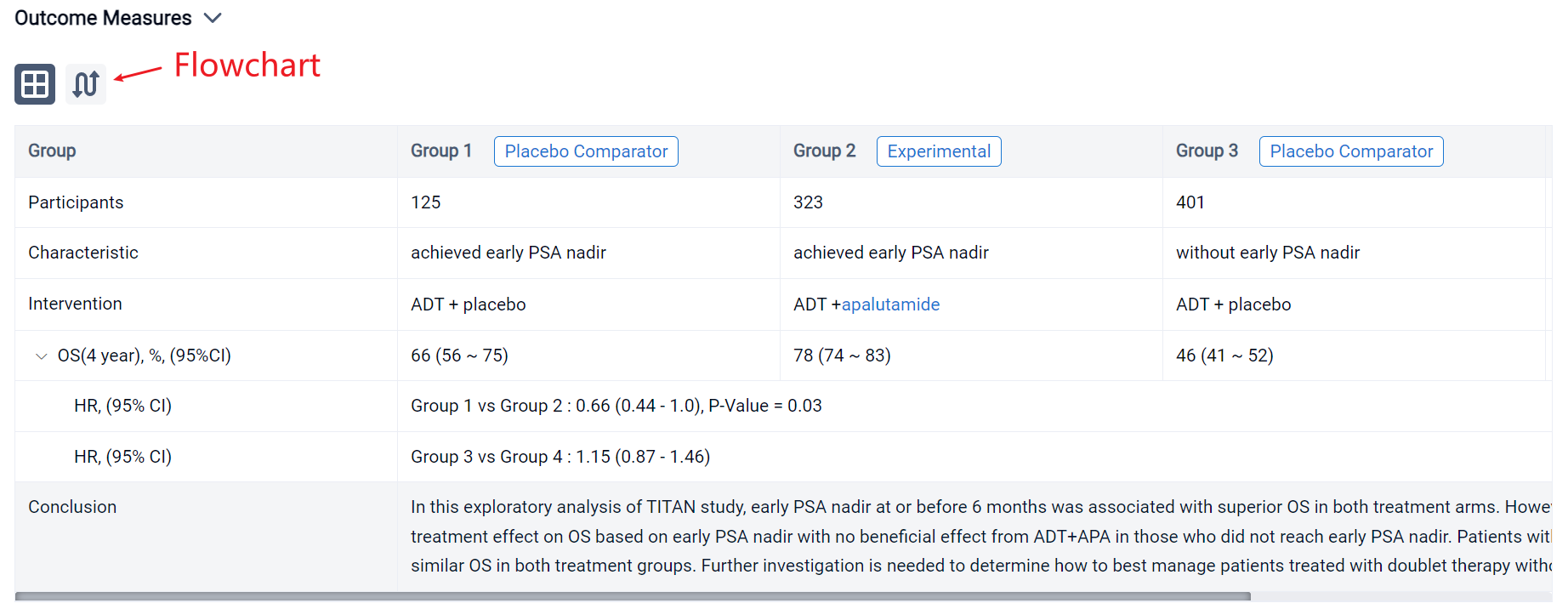
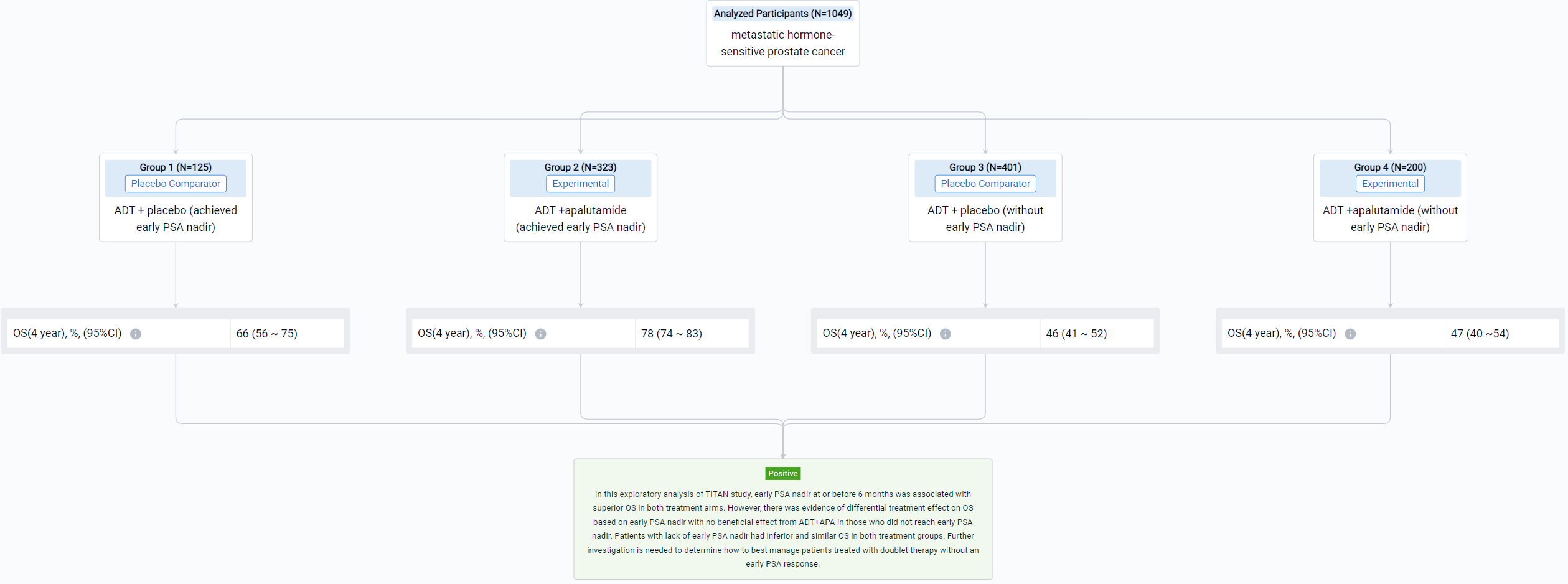
The result showed that 1049 patients were eligible with 526 in the ADT arm and 523 in the ADT+APA arm. Approximately 24% (125/526) patients in the ADT arm and 62% (323/523) patients in the ADT+APA arm achieved early PSA nadir. On landmark analysis, ADT+APA was associated with improved OS among those with an early PSA nadir (HR: 0.66; 95% CI: 0.44-1.00) but no improvement in OS among those without an early PSA nadir (HR: 1.15 [0.87-1.46]) with differential treatment effect between groups stratified by early PSA nadir by 6 months (p=0.03). Adjusted 4-year OS for patients who reached PSA nadir was 66% (95% CI: 56-75) in the ADT alone arm and 78% [74-83] in the ADT+APA arm while for patients who did not reach PSA nadir, adjusted 4-year OS was 46% [41-52] and 47% [40-54] in the ADT alone and ADT+APA arm, respectively. Numbers needed to treat (NNT) was 7.8 (95% CI: 6 to 15) for the overall landmark population, 7.7 [5 to 36] for patients with PSA nadir by 6 months, and 55 [10 to -4] for those without PSA nadir, respectively.
It can be concluded that in this exploratory analysis of TITAN study, early PSA nadir at or before 6 months was associated with superior OS in both treatment arms. However, there was evidence of differential treatment effect on OS based on early PSA nadir with no beneficial effect from ADT+APA in those who did not reach early PSA nadir. Patients with lack of early PSA nadir had inferior and similar OS in both treatment groups. Further investigation is needed to determine how to best manage patients treated with doublet therapy without an early PSA response.
How to Easily View the Clinical Results Using Synapse Database?
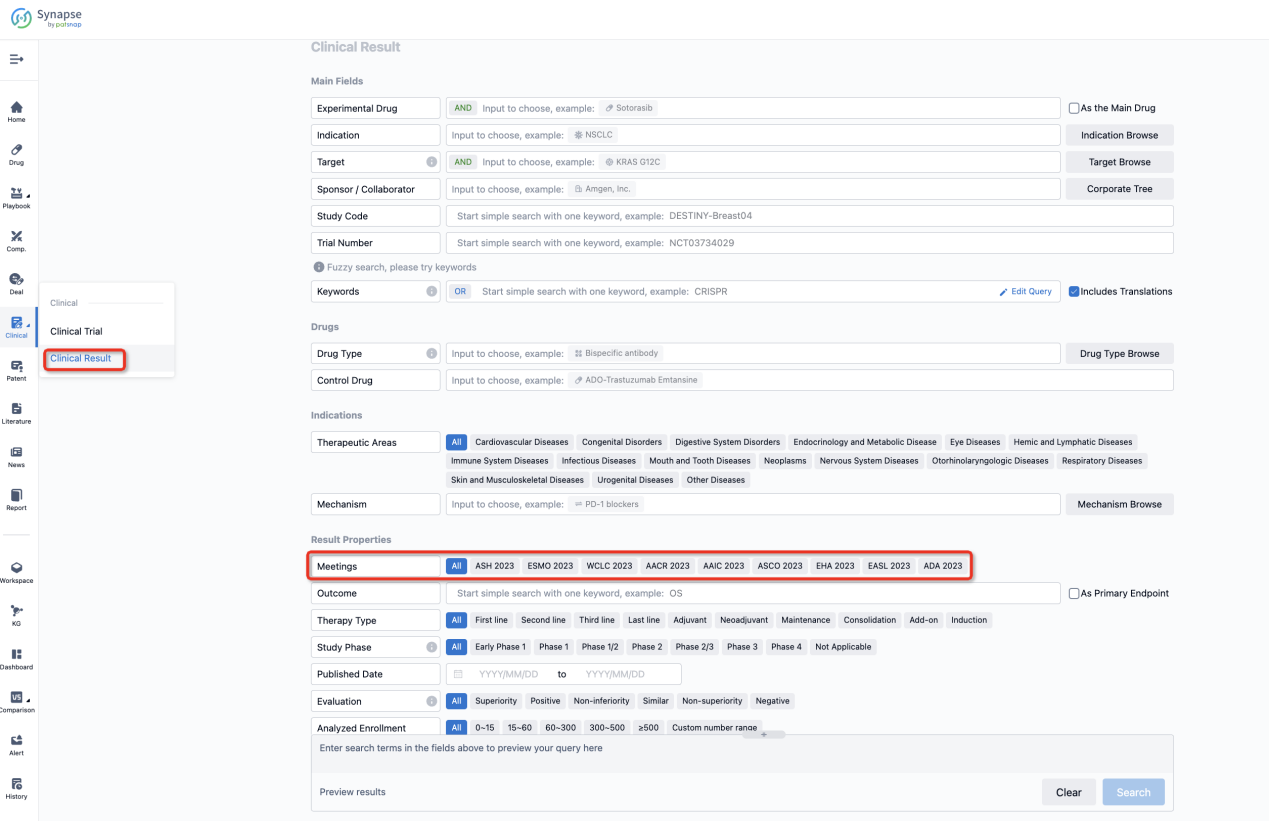
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
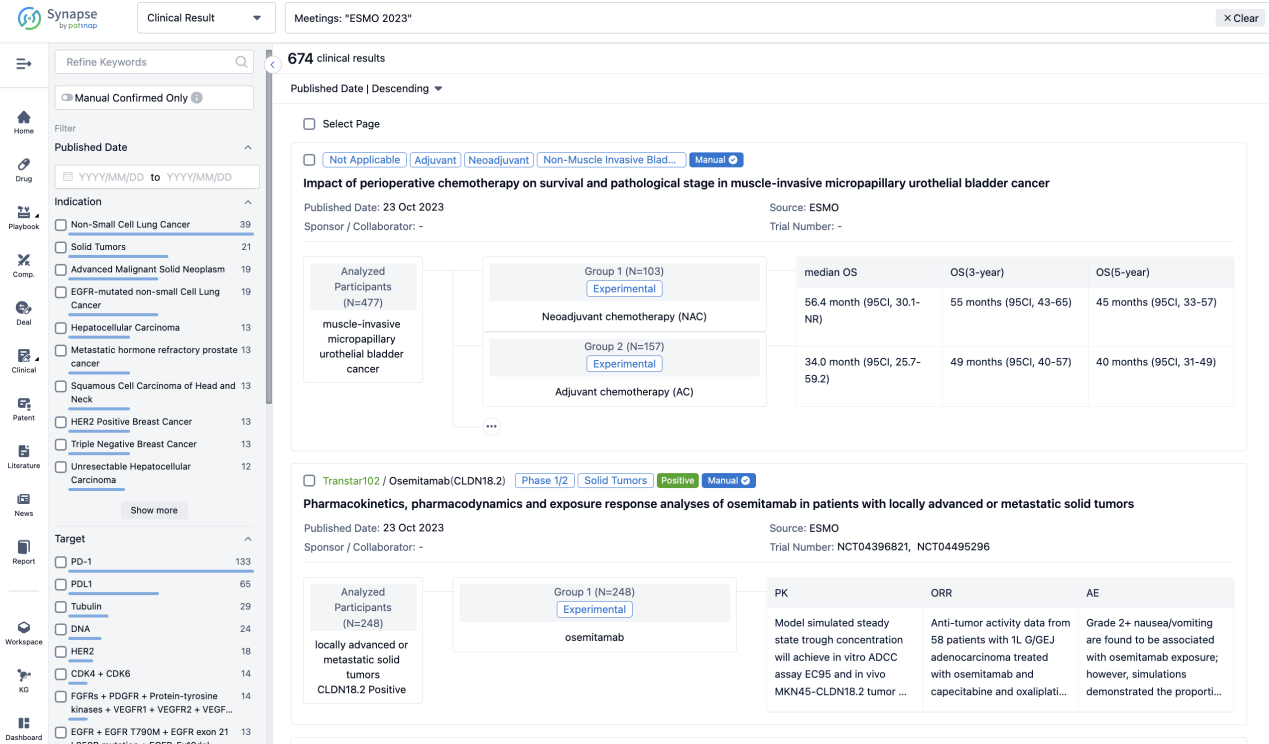
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!