Citius Pharma Resubmits LYMPHIR™ BLA for Treatment of Adult Relapsed/Refractory CTCL
Citius Pharmaceuticals, Inc. has declared that they have refiled their Biologics License Application with the U.S. Food and Drug Administration concerning their product, LYMPHIR™ (denileukin diftitox), which is an IL-2-driven immunotherapeutic agent. This resubmission pertains to its use as a treatment option for individuals suffering from cutaneous T-cell lymphoma who have not responded to or relapsed following initial systemic treatment.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Following continuous dialogue with the FDA in response to a Complete Response Letter issued on July 28, 2023, a revised submission has been made. Citius is confident that it has successfully implemented the required improvements in product examination and production oversight highlighted within the correspondence.
There have been no concerns related to safety or effectiveness presented, nor is there a need for further clinical studies. Anticipating adherence to the Center for Drug Evaluation and Research's schedule, the FDA's recognition of the resubmission and a subsequent allocation of a Prescription Drug User Fee Act (PDUFA) date is anticipated to occur no later than 30 days post-resubmission.
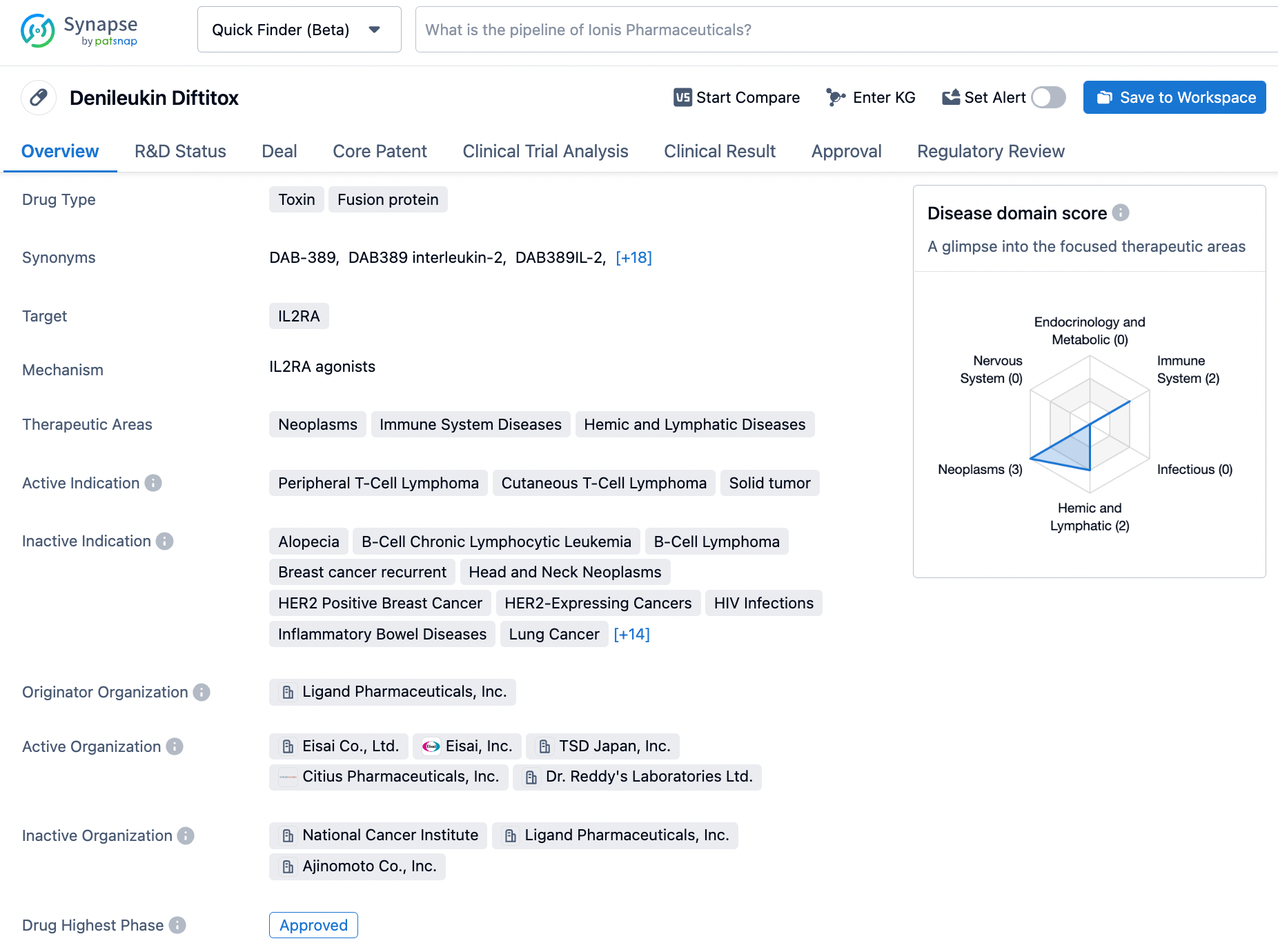
LYMPHIR is a biologic entity, specifically a recombinant fusion protein that marries the interleukin-2 receptor's binding segment with fragments of diphtheria toxin. This compound precisely targets IL-2 receptors on cellular membranes, leading to intracellular diphtheria toxin fragments that disrupt protein production. In the years 2011 and 2013, the FDA endowed LYMPHIR with orphan drug status for addressing PTCL and CTCL respectively.
In the year 2021, the compound denileukin diftitox garnered regulatory sanction in Japan for applying to CTCL and peripheral T-cell lymphoma. Later that same year, Citius secured exclusive licensing, retaining development and marketing rights for LYMPHIR across all territories with the exception of Japan and certain Asian regions.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
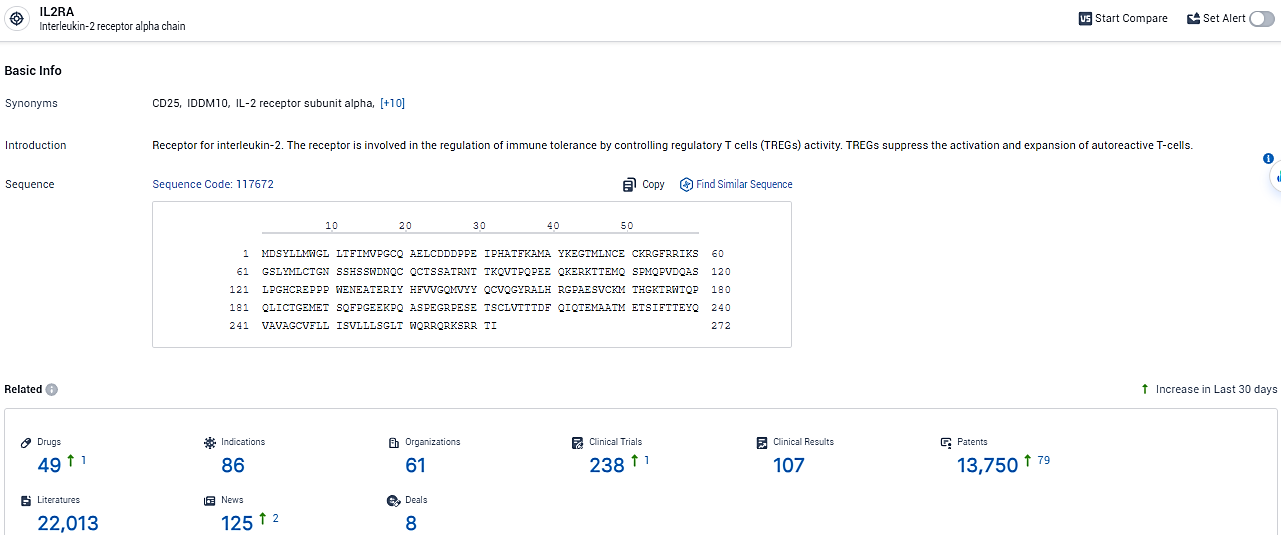
According to the data provided by the Synapse Database, As of February 22, 2024, there are 49 investigational drugs for the IL2RA target, including 86 indications, 61 R&D institutions involved, with related clinical trials reaching 238, and as many as 13750 patents.
Denileukin Diftitox targets the IL2RA receptor and has been approved for the treatment of peripheral T-cell lymphoma, cutaneous T-cell lymphoma, and solid tumors. Its first approval was obtained in the United States in 1999, and it has since been recognized for its potential in addressing unmet medical needs. Denileukin Diftitox's accelerated approval and orphan drug status further emphasize its significance in the pharmaceutical industry.